
Revista Portuguesa de Estomatologia, Medicina Dentária e Cirurgia Maxilofacial
SPEMD - Revista Portuguesa de Estomatologia Medicina Dentária e Cirurgia Maxilofacial | 2025 | 66 (3) | 145-153
Original research
Quality of life and oral health of people undergoing chemotherapy: an observational study
A qualidade de vida e saúde oral de pessoas submetidas a quimioterapia: estudo observacional
a University of Lisbon, Faculty of Dental Medicine, Lisbon, Portugal
Patrícia Flores - patricia.aflores20@gmail.com
Article Info
Rev Port Estomatol Med Dent Cir Maxilofac
Volume - 66
Issue - 3
Original research
Pages - 145-153
Go to Volume
Article History
Received on 19/12/2024
Accepted on 13/06/2026
Available Online on 25/09/2025
Keywords
Original Research
�
Quality of life and oral health of people undergoing chemotherapy: an observational study
A qualidade de vida e sa�de oral de pessoas submetidas a quimioterapia: estudo observacional
�
Patr�cia Flores1* 0009-0008-8075-8562
Teresa Albuquerque1 0000-0002-8539-4648
F�tima Bizarra1 0000-0003-2186-0563
Henrique Lu�s1 0000-0002-1092-7825
Sandra Ribeiro Gra�a1 0000-0001-7970-5270
1 University of Lisbon, Faculty of Dental Medicine, Lisbon, Portugal.
�
�
Article history:
Received 19 December 2024
Accepted 13 June 2025
Available online 24 September 2025
�
Abstract
Objectives: To investigate the impact of chemotherapy on the oral health and quality of life of patients at the Oncology Department of Vila Franca de Xira Hospital.
Methods: This cross-sectional observational study, with an analytical component, involved 76 patients aged between 18 and 65 in the hospital's Oncology Department. A questionnaire was administered to participants about changes in oral health and oral hygiene habits during chemotherapy, and to doctors to collect medical information related to chemotherapy. Data were analyzed using IBM SPSS Statistics V.27, using descriptive and inferential methods at a 5% significance level (p<0.05).
Results: After the start of chemotherapy, 89.5% of respondents experienced at least one oral alteration; the most common conditions that negatively impacted quality of life were edema of the mouth (p=0.050), trismus (p=0.040), mucositis (p=0.008), ulcers (p=0.008), and oral pain (p=0.003). More than 50% of respondents had an unsatisfactory oral health-related quality of life. Only the monoclonal antibody protocol was significantly associated (p=0.035) with oral symptoms, while the anthracycline group protocol was associated with a higher frequency of oral manifestations.
Conclusions: The participants in the study had a high prevalence of self-reported oral alterations resulting from chemotherapy, with an impact on quality of life related to oral health, and there were differences between protocols. Understanding the signs and symptoms can help improve quality of life, enabling preventive actions.
Keywords: Chemotherapy, Chemotherapy protocols, Mucositis, Oral health, Oral manifestations, Quality of life, Xerostomia.
�
Resumo
Objetivos: Investigar o impacto da quimioterapia na sa�de oral e na qualidade de vida dos pacientes do Servi�o de Oncologia do Hospital de Vila Franca de Xira.
M�todos: Este estudo observacional transversal, com componente anal�tica, envolveu 76 pacientes com idades entre os 18 e 65 anos no Servi�o de Oncologia do hospital. Foi aplicado um question�rio aos participantes sobre mudan�as na sa�de oral e h�bitos de higiene oral durante a quimioterapia e aos m�dicos para recolha de informa��es m�dicas relacionadas com a quimioterapia. Os dados foram analisados no IBM SPSS Statistics V. 27, atrav�s de an�lise descritiva e inferencial para um n�vel de signific�ncia de 5% (p<0,05).
Resultados: Ap�s o in�cio da quimioterapia, 89,5% dos entrevistados sofreram pelo menos uma altera��o oral; as condi��es mais comuns que impactaram negativamente a qualidade de vida foram o edema da boca (p=0,050), trismo (p=0,040), mucosite (p=0,008), �lceras (p=0,008) e dor oral (p=0,003). A qualidade de vida relacionada com a sa�de oral foi insatisfat�ria em mais de 50% dos entrevistados. Apenas o protocolo dos anticorpos monoclonais est� significativamente associado (p=0,035) aos sintomas orais, enquanto o protocolo do grupo das antraciclinas est� associado a uma maior frequ�ncia de manifesta��es orais.
Conclus�es: Os participantes do estudo apresentam uma alta preval�ncia de altera��es orais autorrelatadas decorrentes da quimioterapia, com impacto na qualidade de vida relacionada com a sa�de oral, havendo diversas entre protocolos. Compreender os sinais e sintomas pode ajudar a melhorar a qualidade de vida, possibilitando a��es preventivas.
Palavras-chave: Quimioterapia, Protocolos de quimioterapia, Mucosite, Sa�de oral, Manifesta��es orais, Qualidade de vida, Xerostomia.
�
Introduction
Cancer survival rates have increased over the last few decades.1 As a result, it has become imperative to consider the patients� quality of life (QoL), of which oral health is an integral part. Oral complications are an underappreciated result of antineoplastic therapies that affect the QoL of patients undergoing chemotherapy.2
Cancer is characterized by the uncontrolled growth of cells with DNA mutations that have spread to the circulatory and lymphatic systems. It is the third leading cause of death in adults.3, 4 Around 70% of cancer patients will receive chemotherapy during treatment, and around 40% will develop oral complications due to intense immunosuppression.< 5 - 7 Treatment options are conditioned by site, stage, and comorbidity of the disease, but can also include surgery, radiotherapy, or the administration of drugs, such as hormone therapy, targeted therapy, or a combination of these.8
Chemotherapy uses cytostatic and cytotoxic agents to prevent the rapid division and/or destruction of malignant cells and is administered in cycles of intense treatment followed by recovery.2 Due to the high oral sensitivity to the toxic effects of chemotherapy and the rapid rate of cell development and renewal, which makes the oral mucosa very vulnerable, lesions in the oral cavity are frequent.7 Chemotherapy-induced low immunity also leads to oral lesions unknown to the patient or family members, depending on the type and dosage of chemotherapy agents received.7 The oral effects of the numerous protocols used in chemotherapy have not yet been described in the literature.
Some chemotherapy protocols are more stomatotoxic than others, and protocols with a greater number of manifestations may cause worse QoL. Likewise, side effects depend on the stage of treatment. In more intense stages, it is common for various oral pathologies to appear in high prevalence and severity, according to the type of tumor, protocol dosage, number and duration of cycles, patient�s age, and oral hygiene habits before and after therapy.6"> 7, 8
Chemotherapy can damage the oral mucosa directly, through the secretion of the chemotherapeutic agent in saliva, or by systemic circulation, which exposes the drug topically to the oral environment.9 Moreover, it causes nausea, vomiting, weight loss, fatigue, pain, and oral symptoms, especially bleeding, discomfort, xerostomia, mucositis, gingivitis, and dysgeusia, due to the impairment of the immune system and/or the hematopoietic system.9 In turn, the oral symptoms induced by severe immunosuppression interfere with the results of medical therapy, such as systemic complications that increase morbidity, hospitalization time, treatment costs, and QoL.9
QoL is related to health and is considered one of the parameters for assessing the impact of antineoplastic treatment on patients, together with disease-free survival and freedom from cancer recurrence.10 It is significantly influenced by oral health and oral symptoms during and after treatment.11
Oral health assessment before treatment provides a prognosis of residual effects, including periodontal assessment, because the risk of infection and bleeding increases dramatically with treatment due to hematologic changes. Loss of tissue integrity, mucositis, dietary changes, and poor oral hygiene can cause oral problems, affecting QoL and the continuation of therapy. In addition, the association between salivary disorders, changes in microflora, and myelosuppression can lead to gingival bleeding, infections, and discomfort.12 Finally, the patient should be assessed regularly post-therapy to identify risks, estimate cariogenic potential, and promote preventive measures. The healthcare professional can develop a treatment plan tailored to the patient�s needs, preventing and mitigating the incidence of oral complications and, consequently, improving QoL.12
The multidisciplinary oral health team is crucial in the diagnosis, treatment, and rehabilitation of cancer and malignant lesions. They are responsible for identifying alterations, motivating and educating for good oral hygiene practices, and preventing complications.
The aim of this study is to assess oral health-related quality of life (OHRQoL) in patients undergoing chemotherapy and evaluate the association between: self-reported oral changes and the start of chemotherapy; patients� oral hygiene practices and changes after the start of therapy; oral health variables and OHRQoL; and therapeutic protocol, type of cancer, and cycles and OHRQoL.
Material and methods
A cross-sectional observational study with an analytical componente was developed, including participants aged between 18 and 65 years, of all genders, ethnicities, and social backgrounds. Participants had to be able to provide informed consente and were undergoing cancer chemotherapy at the Oncology Department at Hospital Vila Franca de Xira in Portugal. This hospital�s Ethics Committee approved the study.
The exclusion factors applied were not using chemotherapy, having only undergone surgery, an uncertain diagnosis, remission, advanced or cured stages, and salivary diseases. Age range limits were used to minimize the influence of confounding factors such as polypharmacy, aging, and chronic diseases. This approach helps ensure a more homogeneous sample, where the effects of chemotherapy protocols on oral health are less likely to be skewed by these additional variables.
The author drew up an oral health questionnaire based on the literature,9, 13, 14 composed of 39 items in total: 17 questions for patients, 8 questions for medical oncologists, and the Oral Health Impact Profile-14 (OHIP-14) questionnaire.15 Face validity of the questionnaire was carried out prior to its application.
The OHIP-14 is the indicator used to assess the impact of oral health on QoL, measuring the individual�s perception of how oral imbalances affect social life and well-being.15 The OHIP-14 was used to classify OHRQoL as good (from 0 to 10), average (from 11 to 20), poor (from 21 to 30), and very poor (above 30).15 The higher the OHIP-14 value, the worse the OHRQoL.
The study followed the international ethical standards of the World Medical Association and the Declaration of Helsinki.
Potential participants received a consent document explaining the study, procedures, objectives, and guaranteeing the confidentiality and privacy of their data. Only participants who properly signed the consent document were included. Confidentiality of all participant information was guaranteed throughout the data collection, processing, and presentation.
Statistical analysis was carried out using the IBM SPSS� 26.0 program, through descriptive and inferential analysis of the variables, looking for associations between oral health, chemotherapy, and other variables. Normality of data distribution was checked using the Kolmogorov-Smirnov test. The Mann-Whitney U and Kruskal-Wallis tests were used to compare variables. The correlation between two variables was assessed by Pearson�s coefficient. All tests were performed at a significance level of 5%.
Results
A total of 76 participants entered this study, 73.7% (n=56) of whom were female. The average age was 48.0 [18-65] (� 9.89) years, with the 48-57 age group being the most representative (n=27; 35.5%). Most of the patients (n=49; 64.5%) had carcinoma of the mammary gland, and 27.6% (n=21) had carcinoma of the digestive tract.
Most participants (n=59; 77.6%) said they brushed their teeth twice daily or more, while 1.3% (n=1) said they never did. Only 15.8% (n=12) performed interproximal hygiene daily, while the majority, 34.2% (n=26), never did so. Less than half (47.4%; n=36) only visited the dentist when needing treatment, and 31.0% (n=27) for dental treatment.
Approximately 1/3 of the sample (n=23; 30.3%) changed their oral hygiene habits after starting chemotherapy, namely, improved brushing (n=13; 56.5%) and using mouthwash (n=12; 52.2%). An equal low number of patients (n=2; 8.7%) reported less than ideal brushing frequency and not brushing on the day of chemotherapy. Regarding their oral health, 46.1% (n=35) rated it unsatisfactory, and only 5.3% (n=4) considered it excellent.
The OHIP-14 results (Table 1) revealed that 44.7% (n=34) of the sample classified their OHRQoL as good. In turn, 35.6% (n=27) considered that they had a very poor or poor OHRQoL.
�
Table 1. Oral health-related quality of life as measured by the Oral Health Impact Profile-14 (OHIP-14).
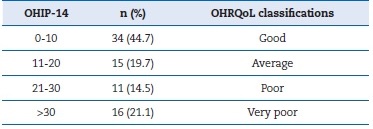
OHRQoL � Oral Health-related Quality of Life.
�
The majority (n=68; 89.5%) of patients reported having had oral manifestations as a result of chemotherapy. The presence of manifestations was correlated with a poorer QoL (p=0.014), as shown in Table 2.
�
Table 2. Relationship between the presence of oral manifestations at the beginning of chemotherapy and the Oral Health Impact Profile-14 (OHIP-14).
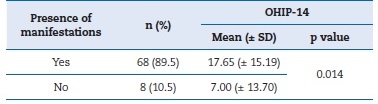
Mann-Whitney U test. SD � standard deviation.
�
Table 3 shows that only 9% of patients received just one protocol. The most commonly used protocol was platinum-alkylating agents (67.1%; n=51), followed by anthracyclines (50.0%; n=31). The taxane antimitotic protocol triggered oral manifestations most often (93.5%), and only monoclonal antibodies showed a significant difference (p=0.035). The anthracyclines protocol caused the highest number of oral manifestations (p=0.008).
�
Table 3. Relationship between the presence and number of oral manifestations and chemotherapy protocols.
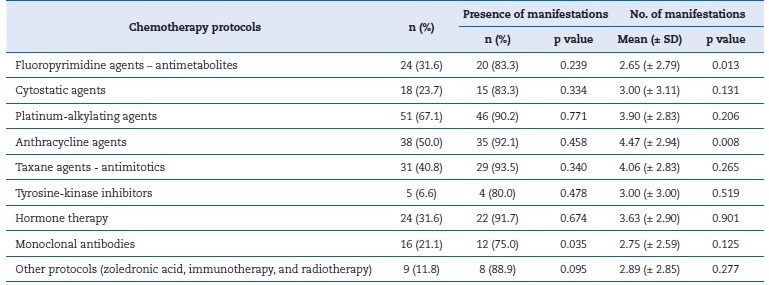
SD � standard deviation.
�
The participants reported 275 oral manifestations, such as tooth sensitivity, xerostomia, nausea, and vomiting. Self-reported manifestations of tooth and mouth pain (p=0.003), ulcers (p=0.008), mucositis (p=0.008), mouth swelling (p=0.050), and trismus (p=0.040) were associated with worse OHIP-14 results and, thus, negatively with QoL (Table 4).
�
Table 4. Relationship between the Oral Health Impact Profile-14 (OHIP-14) and self-reported oral manifestations.
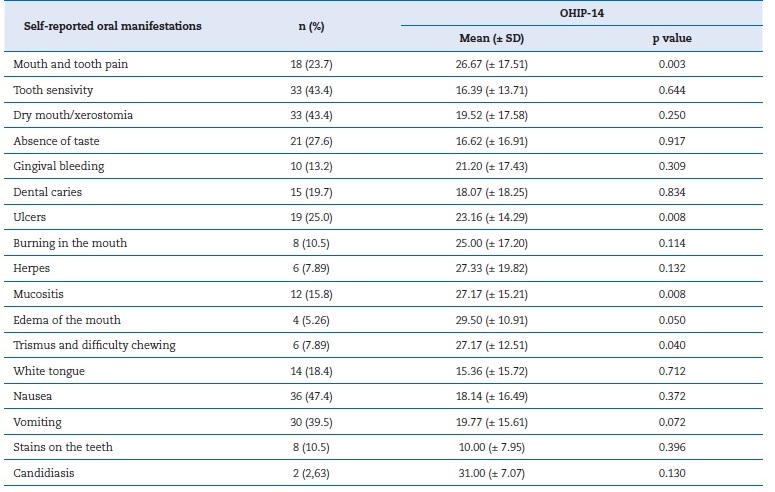
SD � standard deviation.
�
No protocol or number of cycles (p=0.388) associated with OHIP-14 results showed statistically significant differences, but the tyrosine-kinase inhibitors protocol contributed to a worse QoL. Moreover, the higher the OHIP-14 value, the fewer protocols the patient performed (r=-0.101) (Table 5).
�
Table 5. Relationship between the Oral Health Impact Profile-14 (OHIP-14) and chemotherapy variables.
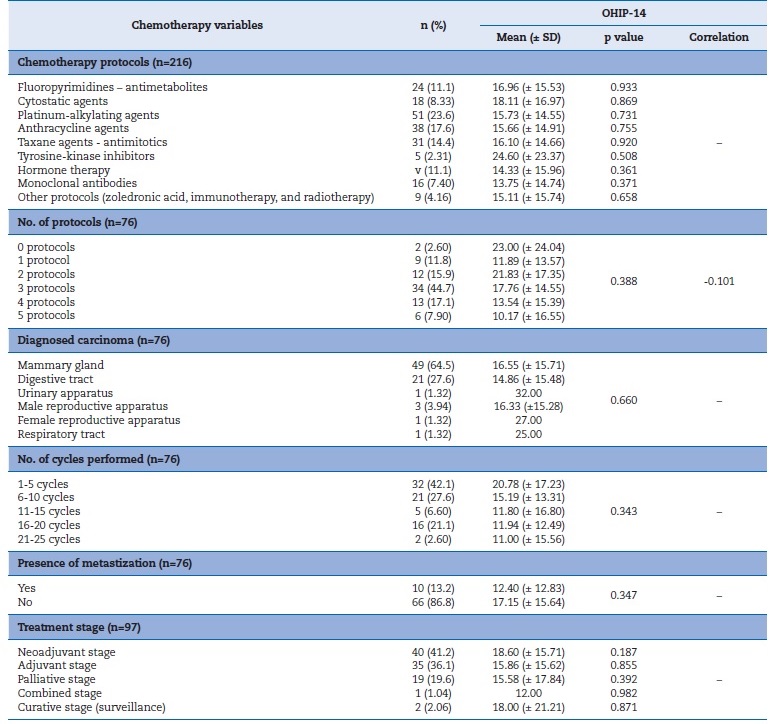
Kruskal-Wallis and Mann-Whitney U tests. SD � standard deviation.
�
The diagnosis, number of cycles, and presence of metastasis showed no significant association with OHIP-14. However, the absence of metastases and the neoadjuvant stage were associated with lower OHRQoL (p= 0.347 and p=0.343, respectively).
Discussion
This study�s sample is small compared to the national oncology population, but includes 90% of the population followed in Oncology at the Hospital de Vila Franca de Xira. The higher female population ratio (2.8 women/1 man) was probably due to the high prevalence of mammary gland carcinoma.
Although this study only covered participants up to 65 years old, the National Oncological Registry of All Tumors in the Resident Population in Portugal, in 2020, found that most new cases (40.0%) occur in the age group between 60 and 74 years old, followed by the group over 75 years old (32.4%).16 The patients had low education levels, with 50.0% having basic education, similar to the 2021 Census (49.7%).17 In that registry, the three most common tumor locations were the breast (7,504 cases), the prostate (5,776 cases), and the lung (4,737 cases).
These findings agree with the present study, in which mammary gland carcinoma was the most common, occurring in 64.5% of participants.16
Oral hygiene before, during, and after chemotherapeutic treatment is essential to reduce or mitigate the onset and development of oral complications, as it reduces the growth of microorganisms and decreases the risk of developing serious diseases.18 When applied systematically, oral care protocols significantly reduce the incidence, severity, and duration of sequelae. However, patients are uninformed about the need for this care and the recurrent evaluation of the oral mucosa.19
In Europe, the frequency of brushing twice daily or more is common among adults, ranging from 75% in Nordic countries to less than 45% in Eastern and Southern countries,20 which reflects a lower adherence to oral health care in Southern countries. However, this study�s results showed positive data, since 77.6% of those surveyed brushed their teeth twice daily or more, a figure higher than in Northern European countries, revealing a concern with maintaining oral health.21 The opposite trend was observed regarding interproximal hygiene habits, with 34.2% never using these means. Therefore, it is essential to reinforce the importance of adopting interproximal methods to reduce oral diseases, which can be exacerbated in cancer patients.18, 19, 22
In the present study, most participants stated that they only sought dental appointments when necessary (47.4%), contrary to the INE 2015 data (17.2%),(23) and 31.0% for dental treatment, which is positive compared to the INE 2015 data (13.3%).23 With the start of chemotherapy, 30.3% of participants changed their oral hygiene habits, improving brushing and the use of mouthwash, similar to the study by Santilal et al.13
The QoL of cancer patients is an indicator of the patient�s response to the disease and treatment. The patient has the best perspective for assessing their own QoL. A study indicates that, along with disease-free survival and freedom from cancer recurrence, QoL is an important parameter for assessing the impact of cancer on individuals.24 Oral manifestations such as mucositis, xerostomia, and opportunistic infections interfere with the results of therapy, leading to systemic complications.
Treatment side effects can be improved and avoided, contributing to a better QoL.10
In the present study, 35.6% of respondents perceived their OHRQoL to be negative, which is a lower number than the one in the study by Pavithran et al.,25 where 45.6% of cancer patients felt that life was less satisfactory due to problems with their teeth and mouth. In Santilal et al.�s study,13 three-quarters of the sample said that oral symptoms impacted their lives, with the most affected QoL associated with the greatest number of oral manifestations, and 22.4% of the sample rated their QoL as worse during chemotherapy.
Chemotherapy has a greater effect on rapidly dividing cells, such as bone marrow, gastrointestinal, and oral mucosa, which are vulnerable to adverse effects.9 14 Oral manifestations as a result of chemotherapy occur in 40% of cancer patients after 1 week of treatment.5, 6, 9, 11, 23, 26 In this study, 89.5% of the participants had some kind of oral manifestation as a result of chemotherapy, which is higher than the findings (58%) of Hespanhol et al.5, but comparable to the findings (89.8%) of Santilal et al.,13 in a Portuguese sample.
The most prevalent oral manifestations in the literature are mucositis, dysgeusia, xerostomia, and candidiasis. In the sample studied, the most prevalent symptoms were tooth sensitivity, xerostomia, ageusia, and ulcers.4, 9, 27 However, the manifestations that were shown to statistically influence OHRQoL were tooth and mouth pain, ulcers, mucositis, mouth swelling, and trismus. Chemotherapy promotes immunosuppression, compromising the immune system and salivary homeostasis, thus predisposing the mucosa to fungal infections. Some studies have detected the presence of candidiasis in 26% of patients.1, 11
Sweeney et al.28 report that candidiasis can occur in plaques, erythematous areas, and chronic atrophic areas.
Dental sensitivity has been linked to low salivary pH and reduced salivary production both during and after treatment.(29) With a frequency of 43.4%, xerostomia and tooth sensitivity were the most common symptoms in the present study.
Xerostomia was a major side effect recorded in 78% of patients assessed by Ariswa et al.31 reported similar findings, and Soares et al.32 reported this symptom in all patients. The literature indicates that antineoplastic medications can change the salivary flow and its constituents, including salivary amylase and IgA,4, 7, 32 both quantitatively and qualitatively.
Aphtous lesions or ulcers were responsible for 4% of the oral manifestations in the studies by Hespanhol et al.5 and Morais et al.9 However, in the present sample, their numbers were higher at 25.0%, presenting a statistically significant difference when related to OHRQoL. Mucositis is reported in the literature in 40 to 76% of cases.4, 7, 33 Conversely, in this study, only 15.8% of the respondentes reported this manifestation. However, this percentage could be higher if the medical team were to diagnose and meticulously record this pathology, or through oral observation of these patients, as initially proposed in the study. It was considered an influencing factor on OHRQoL.
A study carried out by Dib et al.34 stated that trismus occurs when the masticatory muscles undergo fibrosis, limiting the opening of the mouth. This condition interferes with the maintenance of oral hygiene, speech, and nutrition, hindering oral rehabilitation. In this study, around 8% of participants experienced trismus, and it was considered an influencing factor on OHRQoL.
Tooth and mouth pain affects about 15 to 50% of oncologic patients, according to Sim�es et al. and other authors,6 35 similar to the percentage found in the present study. Participants considered it influenced OHRQoL.
Vomiting is the most common symptom in many studies, may present in varying intensity, and may cause mild discomfort to severe imbalance. Besides the associated discomfort, this symptom should also be prevented because of the possibility of impairing the treatment.36 In this study, 39.5% of the participants reported it. However, contrary to the literature, it was not considered an influencing factor of OHRQoL.
According to Hespanhol et al.,5 edema of the mouth may be associated with mucositis and hemorrhage, generating severe discomfort that results in loss of OHRQoL. In this sample, only 5.26% of participants reported it. However, it was considered to influence QoL, coinciding with data in the literature.
With antineoplastic treatment, patients present complications that cause loss of OHRQoL, such as pain, dysphagia, and nutritional impairment due to eating.27 Oral lesions are entry points for opportunistic microorganisms, aggravated by immunosuppression.
The grouping of substances by pharmacological group was based on current oncology guidelines.36, 37 The most stomatotoxic protocols in the literature are fluoropyrimidines, cytostatics, alkylating agents, antimitotics, and anthracyclines, with a greater number of oral manifestations,11, 38, 39 and possibly worse QoL. In this study, fluoropyrimidines and anthracyclines caused the most manifestations, which is in line with the literature.10, 38, 40, 41
The difficulties inherent to studying an oncologic population are multiple and complex, as it is a very diverse population group. The choice of this population is due to their rapidly increasing representation in society and the need to know their oral health status to obtain epidemiological data to support programs that do not exist in Portugal.
This research has limitations, such as the impossibility of observing participants orally due to the pandemic and the sample size (n=76), which does not allow the results to be extrapolated.
The reasons for refusing to participate were poor health and personal temperament, but the medical team�s recommendation to participate increased adherence.39
Self-reporting of manifestations can introduce a memory bias or bias statements in the desired direction. Paper-based health questionnaires induce filling errors, and the wide dispersion of QoL data, with high standard deviations, makes it difficult to distinguish between probable effects and real effects. Moreover, the great variability of therapeutic protocols and cycles made it difficult to obtain statistically significant associations.
The selection bias in choosing the age range of 18 to 65 years may affect the generalizability of the study results to the broader population of oncology patients, as the demographic and clinical characteristics of individuals outside this age range may differ. These differences could influence the response to chemotherapy and, consequently, the oral manifestations observed. The restriction applied to the age range may result in a sample that is less representative of the general population, potentially compromising the applicability of the results to all chemotherapy-treated patients.
The QoL questionnaire used may not accurately represente the experiences of cancer patients. One of the alternatives could be the EORTC QLQ-OH15 from the Oral Health Module of the European Organization for Research and Treatment of Cancer.42
The results of this research show that the various chemotherapy protocols have different clinical impacts, which consequently affect the patient�s QoL. Anticipating oral manifestations, thus preventing suffering, pain, and discomfort, can improve the QoL of patients undergoing chemotherapy. In addition, patients� unmet oral needs may not only affect their QoL, but also compromise the short-, medium-, and long-term success of chemotherapy treatment.
More clinical prevalence studies are needed to assess the presence of self-reported manifestations by patients. However, clinical records should also be improved to enable the creation of clinical protocols appropriate to the patient�s medical situation.
In addition, the presence of an oral health professional in oncology services would be very positive for supporting and assisting in diagnosing, treating, or mitigating the oral manifestations present.
Including oral health protocols before and during chemotherapy is essential to prevent the most common oral complications arising from the treatment�s side effects, such as oral mucositis and xerostomia (dry mouth), to improve QoL, to maintain proper oral hygiene procedures, and to aid in the rapid recovery of oral lesions. Given the significant impact of oral complications on the QoL of patients undergoing antineoplastic treatments, pre-chemotherapy consultations are essential for assessing and managing the patient�s oral health, identifying infection sites, and providing guidance on preventive measures such as proper hygiene and hydration. Additionally, regular follow-up during treatment allows for monitoring and managing oral complications, such as mucositis and xerostomia, improving treatment adherence and overall well-being.
The implementation of structured protocols promotes effective and essential interdisciplinary care. The protocol should include pre-chemotherapy consultations with a comprehensive oral health assessment, the implementation of preventive measures, and instructions on oral hygiene and diet. During chemotherapy, the patient should be monitored every 3 to 4 weeks to detect and treat potential oral complications.
After the completion of chemotherapy, it is essential to conduct a new oral health assessment and monitor long-term effects and the recovery of oral lesions and dental problems, with follow-up consultations every 3 to 6 months.
Conclusions
The present study demonstrated the high prevalence of oral manifestations related to chemotherapy. Of the patients observed, 89.5% presented some oral manifestation associated with chemotherapy, with the anthracyclines protocol causing the highest number of manifestations.
The presence of oral health professionals in multidisciplinar teams, including the oncology team, is deemed relevant for them to actively participate in the prevention and treatment of oral lesions resulting from chemotherapy, both in the initial stage of diagnosis and during and after therapy. In turn, oral health teams should be aware that patients with cancer require specific oral care at regular intervals and develop a treatment plan appropriate to the conditions presented, to improve their QoL.
It is essential to provide patients with clear and specific information about carcinoma and its impact on oral health, along with preventive measures such as maintaining proper oral hygiene, hydration with saliva substitutes or stimulants, managing mucositis and oral infections, regular visits to oral health professionals, and avoiding smoking and excessive alcohol consumption. These actions help minimize adverse effects and improve the patient�s QoL.
Further studies, preferably longitudinal, with larger samples, are needed to follow the patient during and after therapy, contributing to a better knowledge of this issue in Portugal and allowing the creation of oral health programs for cancer patients that are more interventive and bring higher gains in health.
Preventive measures are essential in the oral health management of patients undergoing chemotherapy. These include maintaining rigorous oral hygiene practices, such as proper tooth brushing and using dental floss, as well as ensuring consistente hydration to mitigate the effects of xerostomia by using saliva substitutes or stimulants when necessary. The prevention of infections and inflammation is equally critical, with recommendations for appropriate mouth rinses and minimization of trauma to the oral mucosa. Priority should also be given to managing mucositis, alongside the adoption of a balanced diet that emphasizes soft foods while avoiding potentially irritating substances. Furthermore, dental consultations should be conducted before, during, and after oncological treatment to address sources of infection, such as caries and periodontitis, and to educate patients about potential oral signs and symptoms associated with chemotherapy.
�
References
1. Wong HM. Oral complications and management strategies for patients undergoing cancer therapy. ScientificWorldJournal. 2014;2014:581795.
2. Moreira A. Complica��es Orais da radioterapia e quimioterapia-implica��es na qualidade de vida. [master dissertation]. Porto (Portugal): Faculdade de Medicina Dent�ria da Universidade do Porto; 2016.
3. Figueiredo PBA, Nogueira AJS. Preval�ncia de Neoplasia, C�rie e Gengivite em Pacientes Pedi�tricos no Munic�pio de Bel�m. Pesqui Bras Odontopediatria Clin Integr. 2013;13:141-6.
4. Ara�jo TLC, Mesquita LKM, Vitorino RM, Macedo AKMN, Amaral RC, Silva TF. Manifesta��es bucais em pacientes submetidos a tratamento quimioter�pico Rev Cubana Estomatol. 2015; 52: 16-21.
5. Hespanhol FL, Tinoco EMB, Teixeira HGC, Falabella MEV, Assis NMSP. Manifesta��es bucais em pacientes submetidos � quimioterapia. Ci�nc Sa�de Coletiva. 2010;15(suppl1):1085-94.
6. Faza JFG, Brum SC. The influence of chemotherapy on oral healthy. Revista Pr�-UniverSUS. 2018;9:81-9.
7. Martins AC, Ca�ador NP, Gaeti WP. Complica��es bucais da quimioterapia antineopl�sica. Acta Scientiarum. 2002;24:663-70.
8. Choi WH, Cho J. Evolving clinical cancer radiotherapy: Concerns regarding normal tissue protection and quality assurance. J Korean Med Sci. 2016;31(Suppl 1):S75-87.
9. Morais AMD, Honda R, Lopes CRP, Concei��o L, Felipe LCS, Milhomem C. Estudo das manifesta��es bucais de pacientes tratados com quimioterapia. J Orofac Invest. 2017;4:49-59.
10. Villar RR, Fern�ndez SP, Garea CC, Pillado MTS, Barreiro VB, Martin CG. Quality of life and anxiety in women with breast cancer before and after treatment. Ver Latino-Am Enfermagem. 2017;25:e2958.
11. Kreuger MRO, Salvoldi LW, Hoffmann S, Dielogi NM. Complica��es Orais em pacientes em tratamento quimioter�pico na UNACON no munic�pio de Itaja�/SC. Ver Fac Odontol Lins (Impr). 2009;21:39-47.
12. Fonseca ISS, Moura LC, Melo IS, Rosa MPRS. Qualidade de vida do paciente quimioter�pico: uma revis�o de literatura entre 2002 a 2012. Interfaces Cient�ficas-Sa�de e Ambiente. 2015;3:25-38.
13. Santilal J, Gra�a S. Oral manifestations in patients with diferent oral health behaviors submitted to chemotherapy: a preliminary study. Rev Port Estomatol Med Dent Cir Maxilofac. 2019;60:118-24.
14. Reis S. Higiene oral na pessoa com doen�a hematooncol�gica a realizar quimioterapia [Master dissertation]. Coimbra (Portugal): Escola Superior de Enfermagem de Coimbra; 2012.
15. Afonso A, Silva I, Meneses R, Frias-Bulhosa J. Qualidade de vida relacionada com a sa�de oral: valida��o portuguesa de OHIP-14. Psicologia, Sa�de & Doen�as. 2017;18:374-88.
16. Instituto Portugu�s de Oncologia do Porto Francisco Gentil � EPE. Registo Oncol�gico Nacional de Todos os Tumores na Popula��o Residente em Portugal, em 2020. Available from: https://ron.min-saude.pt/media/2223/ron-2020.pdf. Accessed 19 December 2024.17. INE � Instituto Nacional de Estat�stica. Censos 2021 � Resultados definitivos. Available from: https://censos.ine.pt/ xportal/xmain?xpgid=censos21_produtos&xpid=CENSOS21&xlang=pt. Accessed 26 May 2025
18. INE � Instituto Nacional de Estat�stica. Censos 2011 � Resultados definitivos. Available from: https://www.ine.pt/ngt_server/attachfileu.jsp?look_parentBoui=156749170&att_display=n&att_download=y. Accessed 26 May 2025
19. Albuquerque ILS, Camargo TC. Preven��o e tratamento da mucosite oral induzida por radioterapia: revis�o de literatura. Rev Bras Cancerol. 2007;53:195-209.
20. Eilers J. Nursing interventions and supportive care for the prevention and treatment of oral mucositis associated with cancer treatment. Oncol Nurs Forum. 2004;31(4 Suppl):13-23.
21. Eaton KA, Carlile MJ. Tooth brushing behaviour in Europe: opportunities for dental public health. Internacional Dental Journal. 2008;58(Suppl 5):287-93.
22. Palmela P. Guidelines para cuidados de sa�de oral em doentes oncol�gicos. Lisboa: Servi�o de Estomatologia do Hospital de Santa Maria, 2010.
23. INE � Instituto Nacional de Estat�stica. Inqu�rito Nacional de Sa�de. November 2015. Available from: https://www.ine.pt/xportal/xmain?xpid=INE&xpgid=ine_publicacoes&PUBLICACOESpub_boui=263714091&PUBLICACOESmodo=2&xlang=pt. �Accessed 26 May 2025
24. Marrs JA. Care of Patients with Neutropenia. Clin J Oncol Nurs. 2006;10:164-6.
25. Pavithran S, Sreeleksmi MV, Sreelekshmi R. Oral-Health related quality of life of patients in chemotherapy. Biomedical & Pharmacology Journal. 2010;13:107-18.
26. Troles T. Estudo comparativo da qualidade de vida antes e ap�s a reabilita��o com implantes dent�rios [Master dissertation]. Caparica: Instituto Universit�rio Egas Moniz. 2018.
27. Silva P. Mucosite oral em pacientes submetidos a quimioter�picos [Master dissertation]. Instituto Universit�rio de Ci�ncias da Sa�de. 2018.
28. Sweeney MP, Bagg J, Baxter WP, Aitchison TC. Oral disease in terminally ill cancer patients with xerostomia. Oral Oncol. 1998;34:123-6.
29. Garg AK, Malo M. Manifestations and treatment of xerostomia and associated oral effects secondary to head and neck radiation therapy. J Am Dent Assoc. 1997;128:1128-33.
30. Ariswa EAL, Silva CMOM, Cardoso CAC, Lemos NRP, Pinto MC. Efeitos colaterais da terapia antitumoral em pacientes submetidos � quimio e � radioterapia. Rev Bioci�n, Taubat�. 2005;11:55-61.
31. Petitto J. Complica��es e sequelas da radioterapia nos c�nceres da cavidade. In: Brand�o L et al. Cirurgia de cabe�a e pesco�o. S�o Paulo: Roca. 1998; 1: 115-6.
32. Soares C. Princ�pios da radioterapia: complica��es no diagn�stico e tratamento do c�ncer em cabe�a e pesco�o. In: Carvalho A, et al. Simp�sio de C�ncer, S�o Paulo: Instituto do C�ncer Arnaldo Vieira de Carvalho. 1999; 1: 21-3.
33. Mravak-Stipetić M. Xerostomia - diagnosis and treatment. Rad 514 Medical Sciences. 2012;38:69-91.
34. Dib LL, Gon�alves RC, Kowalski LP, Salvajoki JV. Abordagem multidisciplinar das complica��es orais da radioterapia. Rv Assoc Paul Cir Dent. 2000;54:391-6.
35. Sim�es CA, Castro JF, Cazal C. Candida Oral como Fator Agravante da Mucosite Radioinduzida. Revista Brasileira de Cancerologia. 2011;57:23-9.
36. IPO Porto. Quimioterapia � Guia de Orienta��o. 2015. Available from: https://www.ipoporto.pt/dev/wp-content/uploads/2017/08/DSN20150006-IPO-Guia-Quimioterapia-E.01.pdf.� Accessed 25 May 2024.
37. Neto MC, Hamerschlack N, Ribeiro AA, Guendelmann AK, dos Santos VA. Guia de Protocolos e Medicamentos para Tratamento em Oncologia e Hematologia. 1st ed. S�o Paulo: Hospital Albert Einstein, 2013. p. 516-520.
38. Morais EF, Lira JAS, Macedo RAP, Santos KS, Elias CTV, Morais MLSA. Oral manifestations resulting from chemotherapy in children with acute lymphoblastic leukemia. Braz J Otorhinolaryngol. 2014;80:78-85.
39. Bensadoun RJ, Magn� N, Marcy PY, Demard F. Chemotherapy and radiotherapy induced mucositis in head and neck cancer patients: new trends in pathophysiology, prevention and treatment. Eur Arch Otorhinolaryngol. 2001;258:481-7.
40. Scherr A, Peixoto A, Soares W. Protocolo Estadual de Quimioterapia Antineopl�sica. Oncologia Cl�nica. Aracaju: Funda��o Estadual de Sa�de- Funesa, 2016. p. 116-20.
41. WHO. Oral healthy surveys. Basic methods. World Health Organization, Geneva. 2013.
42. Hjermstad MJ, Bergenmar M, Bjordal K, Fisher SE, Hofmeister D, Montel S, et al. International field testing of the psychometric properties of an EORTC quality of life module for oral health: the EORTC QLQ-OH15. Support Care Cancer. 2016;24:3915-24.
�
Patr�cia Flores
E-mail address: patricia.aflores20@gmail.com
�
CRediT authorship contribution statement
Patr�cia Flores: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing � original draft, Writing � review & editing. Teresa Albuquerque: Conceptualization, Formal analysis, Methodology, Writing � original draft. F�tima Bizarra: Conceptualization, Formal analrev ysis, Methodology, Resources, Writing � original draft. Henrique Lu�s: Conceptualization, Formal analysis, Validation, Visualization, Writing � original draft. Sandra Ribeiro Gra�a: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing � original draft, Writing � review & editing.
�
Conflict of interest
The authors have no conflicts of interest to declare.
�
Ethical disclosures
Protection of human and animal subjects. The authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of data. The authors declare that they have followed their work center protocols on access to patient data and for its publication.
Right to privacy and informed consent. The authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
�
1646-2890/� 2025 Sociedade Portuguesa de Estomatologia e Medicina Dent�ria. Published by SPEMD.
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
