
Revista Portuguesa de Estomatologia, Medicina Dentária e Cirurgia Maxilofacial
SPEMD - Revista Portuguesa de Estomatologia Medicina Dentária e Cirurgia Maxilofacial | 2023 | 64 (1) | 50-54
Case report
Metastasis to the sublingual gland of metaplastic breast carcinoma – a case report
Metástase para a glândula sublingual de um carcinoma metaplásico da mama – relato de caso
a Centro Hospitalar e Universitário de Coimbra – Serviço de Anatomia Patológica, Coimbra, Portugal
b Faculdade de Medicina da Universidade de Coimbra, Coimbra, Portugal
c Centro Hospitalar e Universitário de Coimbra – Serviço de Estomatologia, Coimbra, Portugal
Vânia Almeida - vaniafi@hotmail.comvaniafi@hotmail.com
Article Info
Rev Port Estomatol Med Dent Cir Maxilofac
Volume - 64
Issue - 1
Case report
Pages - 50-54
Go to Volume
Article History
Received on 30/09/2022
Accepted on 09/03/2023
Available Online on 31/03/2023
Keywords
Clinical Case Report
�
Metastasis to the sublingual gland of metaplastic breast carcinoma � a case report
Met�stase para a gl�ndula sublingual de um carcinoma metapl�sico da mama � relato de caso
�
V�nia Almeida1,2,* 0000-0002-2202-7402
Carlos Salgado3 0000-0002-8243-4413
Pedro Ferraz3 0000-0003-0753-1608
Lina Carvalho1,2 0000-0001-8349-4488
1 Centro Hospitalar e Universit�rio de Coimbra � Servi�o de Anatomia Patol�gica, Coimbra, Portugal
2 Faculdade de Medicina da Universidade de Coimbra, Coimbra, Portugal
3 Centro Hospitalar e Universit�rio de Coimbra � Servi�o de Estomatologia, Coimbra, Portugal
�
�
Article history:
Received 30 September 2022
Accepted 9 March 2023
Available online 30 March 2023
�
Abstract
Metastatic spread to the salivary glands is rare, occurring primarily from head and neck tumors to the parotid gland. So far, only one sublingual gland metastasis has been reported in the literature. We herein report the second case of metastasis to this gland occurring in a woman in her 50s, previously diagnosed with HER2-positive metaplastic breast carcinoma (MpBC) with chondroid differentiation, with lymph node and diffuse organ metastases. The sublingual lesion presented as a tumefaction on the floor of the mouth, causing masticatory discomfort. Histologically it was formed by epithelial cells with high-grade cytomorphological features with a large central area of chondroid differentiation. The case was clinically and pathologically challenging, given the rarity of the primary MpBC and the improbable secondary location. Alternative primary salivar gland tumors with chondroid differentiation were considered, but ultimately the clinical integration was crucial in establishing the diagnosis of MpBC metastasis to the sublingual gland.
Keywords: Breast carcinoma. Metastasis. Sublingual gland
�
Resumo
As met�stases para as gl�ndulas salivares s�o raras, ocorrendo sobretudo de neoplasias da cabe�a e do pesco�o para a gl�ndula par�tida. At� ao momento apenas uma met�stase na gl�ndula sublingual foi reportada na literatura. Neste trabalho apresentamos o segundo caso, que ocorreu numa mulher na casa dos 50 anos, anteriormente diagnosticada com carcinoma metapl�sico da mama (MpBC) com diferencia��o condroide, e com met�stases ganglionares e org�nicas difusas. A les�o sublingual surgiu como uma tumefa��o no pavimento da boca que causava desconforto na mastiga��o. Histologicamente era formada por c�lulas epiteliais com caracter�sticas citomorfol�gicas de alto grau, com uma �rea central condroide. O caso foi desafiante dada a raridade do tumor prim�rio e o local improv�vel de metastiza��o. Consider�mos tumores prim�rios da gl�ndula salivar com diferencia��o condroide como diagn�sticos alternativos, mas a integra��o cl�nica foi crucial para estabelecer o diagn�stico de met�stase de MpBC para a gl�ndula sublingual.
Palavras-chave: Carcinoma da mama, Met�stase, Gl�ndula sublingual
�
Introduction
Sublingual salivary glands are the smallest of the major salivary glands, located beneath the mucous membrane of the floor of the mouth. These glands can be afflicted by various pathological processes, mostly non-tumoral diseases. Neoplasms of the sublingual salivary glands are unusual, comprising about 1% of all epithelial salivary gland tumors.1
Moreover, metastatic disease to this gland is practically inexistent, with only one case reported in the literature.2 Metaplastic breast carcinoma (MpBC) is a heterogenous subtype of invasive breast carcinoma (IBC) with differentiation of neoplastic epithelium toward squamous or mesenchymal elements, including spindle, chondroid, and osseous cells.3
Most cases have a triple-negative phenotype, meaning a lack of expression of the estrogen receptor (ER), the progesterone receptor (PR), and the human epidermal growth factor receptor 2 (HER2). MpBC accounts for less than 1% of IBCs and occurs mainly in postmenopausal women. It generally takes an aggressive course with a poor prognosis, with 50% of patients developing distant metastasis after primary breast surgery, commonly to the lungs and bones.4, 5 We report the first case of a rare MpBC metastasis to the sublingual salivary gland.
Case report
A female in her 50s was referred to a Stomatology consultation presenting a swelling on the floor of the mouth (Figure 1), causing masticatory and swallowing discomfort. The mass had been growing for the last month since it was initially detected. The patient had a medical history of breast carcinoma diagnosed three years before. At the time, she already had metastatic disease at mediastinal lymph nodes and multiple metastatic nodules located in the lungs, liver, bone, and brain.
�
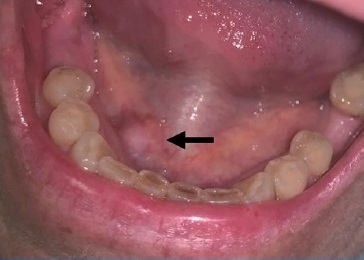
Figure 1. Clinical photograph showing the swelling in the floor of the mouth.
�
On physical evaluation, a painless 1.2-cm firm nodule was palpable sublingually, leading to the presumptive diagnosis of sialolithiasis. The mass was removed and submitted to pathological examination. Grossly, the lesion was firm and grayish with irregular borders (Figure 2). Histopathology showed a malignant lesion comprising neoplastic epithelial cells arranged in small nests with a large central area of chondroid matrix (Figure 3). The epithelial cells were large, with hyperchromatic and pleomorphic nuclei presenting a high mitotic rate (Figure 4). At the edge of the fragment, salivary parenchyma exhibiting ductal ectasia with scant inflammatory cells was identified. On immunohistochemistry, the carcinomatous cells expressed cytokeratins AE1/AE3 and CK7.
�

Figure 2. The surgical specimen: an irregular round mass with a greyish surface and a 1.20-cm diameter.
�
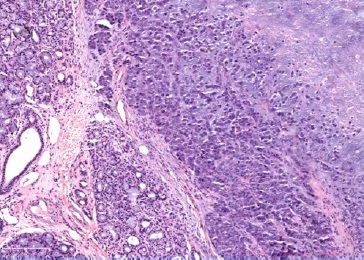
Figure 3. Periphery of the sublingual lesion, showing sublingual parenchyma (left), and tumor with chondroid matrix (right), HE.
�
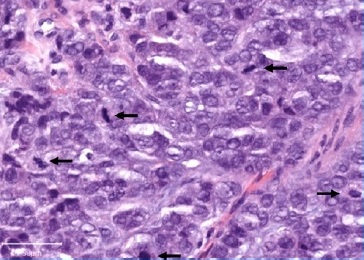
Figure 4. High magnification of the tumoral cells showing high-grade cytological features and several mitoses (arrows), HE.
�
Further investigation of the patient�s medical history revealed that the primary tumor was a HER2-positive MpBC with chondroid differentiation (Figure 5) and positive axillary nodal involvement. She had neoadjuvant treatment (pertuzumab + trastuzumab + docetaxel) followed by modified radical mastectomy with axillary lymph node dissection. The patient then started radiotherapy, adjuvant chemotherapy, and trastuzumab, but the neoplasia progressed under treatment, with disseminated organ metastases.
�
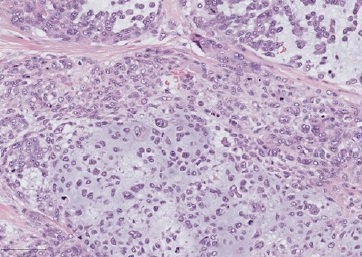
Figure 5. Area of chondroid differentiation in the primary breast carcinoma, HE.
�
The slides from the breast carcinoma were reviewed for morphological and immunohistochemistry comparison with the salivary gland tumor. Both showed negativity for GATA 3, ER, and PR. HER2 was negative in the salivary-gland-located tumor. Based solely on histology, we would have pondered primary salivary tumors with chondroid differentiation. From those, pleomorphic adenoma (PA) is a diagnosis to consider, as it is the most common neoplasia of the salivary glands, although uncommon in the sublingual glands. However, the tumor�s histopathological features conflicted with this hypothesis, particularly the marked nuclear pleomorphism. Moreover, the clinical presentation as a rapidly growing mass was incompatible with the PA diagnosis.
For the malignant counterpart, carcinoma ex-adenoma pleomorphic (CExAP), we would need to have histological evidence or clinical history of a previous PA, which we had not.
Given the patient�s medical record and information on the primary breast tumor�s histological type, we diagnosed metastatic breast carcinoma in the sublingual salivary gland. Immunohistochemistry does not help distinguish this entity from PA and CExAP, which are usually ER and PR negative, with variable GATA3 expression.6, 7
On the follow-up Stomatology appointment, the patient reported improvement from the discomfort associated with mouthfloor tumefaction. She will continue her oncology follow-up care.
Discussion and conclusions
Tumors in sublingual salivary glands are rare, comprising less than 1% of all salivary gland tumors.8, 9 Clinically, they form masses on the floor of the mouth and can be misdiagnosed as other more common diseases in that location, such as ranula, chronic sialadenitis, or sialolithiasis, as happened to our patient.
About 90% of the tumors arising in the sublingual salivary glands are malignant, the most common histological types being adenoid cystic carcinoma, followed by mucoepidermoid carcinoma. Together they account for approximately 70% of malignant sublingual gland tumors.10, 11 Of the rarer benign tumors, PA is the most frequently encountered neoplasia.11
Metastatic spreading to the salivary glands is uncommon and occurs predominantly in the parotid gland. As this major gland has lymph nodes within, secondary parotid malignancies can either occur as lymph node metastases or by infiltration of the parotid parenchyma. Head and neck tumors make up two-thirds of the metastases, while infraclavicular primary tumors are less likely to spread to the parotid.12 These distant metastases derive from numerous primary sites, including about 30 cases of metastasis from breast carcinomas,13 - 20 and are thought to reach the parotid through the thoracic duct or paraspinal venous plexus.18
The other major salivary glands � submandibular and sublingual glands � are much less frequently affected by secondary tumors. Different from the scenario described to the parotid, the head and neck neoplasias are unlikely to metastasize to those glands, and its involvement occurs through extension from the primary tumor or a locally involved lymph node. Secondary involvement of the submandibular gland from infraclavicular primary sites is uncommon. Reported in the medical literature are isolated case reports or small case series, including a few cases originating from primary breast carcinomas. 19, 21 - 27 To our knowledge, only one other case reports secondary deposits in the sublingual gland, after a primary renal clear cell carcinoma.2
In our case, the primary tumor was a rare MpBC with chondroid differentiation. This particular histological subtype of breast carcinoma is rare,28 and, as far as we know, has never been reported to spread to any salivary gland. The high incidence of distant metastases and low nodal involvement rates suggests that the hematogenous route plays an essential role in its dissemination.29 In our case, the patient had both lymph node metastasis and extensive hematogenous dissemination to the lung, liver, bone, and brain at the sublingual metastatic diagnosis.
On pathological examination, the chondroid matrix on the secondary lesion brought diagnostic complexity to the case, as we needed to consider primary salivary tumors with chondroid differentiation as possible diagnoses. Morphology and the clinical setting were crucial to achieving the diagnosis.
Immunohistochemistry did not help distinguish primary from metastatic tumors and showed discordance regarding HER2 status between primary and secondary lesions. The sublingual salivary metastasis showed no HER2 expression on immunohistochemistry, while the primary breast tumor was an exceedingly rare HER2-positive MpBC. Phenotype discordance is considered a frequent event during breast cancer progression.
In particular, HER2 discordance in breast tumors has been reported in up to 15% of cases, mainly transforming from HER2-positive status in the primary tumor to HER2-negative status at metastasis/recurrence sites.30, 31 This conversion is attributed to intratumor heterogeneity and changes in the molecular profile during tumor progression, spontaneously, or following the selection pressure on tumoral clones inflicted by the treatments,32 such as anti-HER2 drugs used in the patient treatment regime.
To the best of our knowledge, this is the first report on MpBC metastasis to the sublingual gland. The diagnosis of this highly unusual case is challenging, both at the clinical and pathological level, considering the rarity of the primary tumor and the peculiarity of the metastatic disease location.
�
References
1. Rinaldo A, Shaha AR, Pellitteri PK, Bradley PJ, Ferlito A. Management of malignant sublingual salivary gland tumors. Oral Oncol. 2004;40:2-5.
2. Ghorra C, Naderi S, Zeid S, Hokayem N, Abboud B. Sublingual gland tumor: Do not forget to rule out a metastasis. ANZ J Surg. 2010;80:669-70.
3. Board. Metaplastic Carcinoma. WHO Classification of Tumours Editorial Board. Breast tumours. Lyon, France: International Agency for Pesearch on Cancer; 2019. p. 134-8.
4. He X, Ji J, Dong R, Liu H, Dai X, Wang C, et al. Prognosis in different subtypes of metaplastic breast cancer: a population-based analysis. Breast Cancer Res Treat. 2019;173:329-41.
5. Reddy TP, Rosato RR, Li X, Moulder S, Piwnica-Worms H, Chang JC. A comprehensive overview of metaplastic breast cancer: clinical features and molecular aberrations. Breast Cancer Res. 2020;22:121.
6. de Souza AA, Altemani A, de Araujo NS, Texeira LN, de Ara�jo VC. Soares AB. Estrogen Receptor, Progesterone Receptor, and HER-2 Expression in Recurrent Pleomorphic Adenoma. Clin Pathol. 2019;12:2632010X19873384.
7. Schwartz LE, Begum S, Westra WH, Bishop JA. GATA3 immunohistochemical expression in salivary gland neoplasms. Head Neck Pathol. 2013;7:311-5.
8. Eveson JW, Cawson RA. Salivary gland tumours. A review of 2410 cases with particular reference to histological types, site, age and sex distribution. J Pathol. 1985;146:51-8.
9. Tian Z, Li L, Wang L, Hu Y, Li J. Salivary gland neoplasms in oral and maxillofacial regions: a 23-year retrospective study of 6982 cases in an eastern Chinese population. Int J Oral Maxillofac Surg. 2010;39:235-42.
10. Andreasen S, Bj�rndal K, Agander TK, Wessel I, Hom�e P. Tumors of the sublingual gland: a national clinicopathologic study of 29 cases. Eur Arch Otorhinolaryngol. 2016;273:3847�56.
11. Lin Y, Wang Y, Zhang H, August M, Xiang X, Zhang F. Sublingual Gland Tumors Worldwide: A Descriptive Retrospective Study of 839 Cases. J Oral Maxillofac Surg. 2020;78:1546�56.
12. Wang H, Hoda RS, Faquin W, Rossi ED, Hotchandani N, Sun T, et al. FNA biopsy of secondary nonlymphomatous malignancies in salivary glands: A multi-institutional study of 184 cases. Cancer Cytopathol. 2017;125:91-103.
13. Cao X-S, Cong B-B, Yu Z-Y. Parotid gland metastasis from carcinoma of the breast detected by PET/CT. Medicine (Baltimore). 2018;97:e10616.
14. Dhia SB, Belaid I, Stita W, Hochlaf M, Ezzairi F, Ahmed SB. Bilateral parotid gland metastasis from a breast invasive ductal carcinoma. J Cancer Res Ther. 2020;16:672-4.
15. Jakharia-Shah A, Wheatley H, Beesley M. Reminder of na important clinical lesson: breast cancer metastasis to the parotid gland. BMJ Case Rep. 2019;12:e226494.16. Jung HK, Lim Y-J, Kim W. Breast Cancer Metastasis to the Parotid: A Case Report with Imaging Findings. Am J Case Rep. 2021;22:e934311.
17. Bohli M, Tebra S, Jaffel H, Bouaouina N. Parotid Gland Metastasis from Breast Origin. J Clin Case Rep. 2018;8:1139.
18. Nuyens M, Sch�pbach J, Stauffer E, Zb�ren P. Metastatic disease to the parotid gland. Otolaryngol Neck Surg. 2006;135:844-8.
19. Seifert G, Hennings K, Caselitz J. Metastatic tumors to the parotid and submandibular glands--analysis and differential diagnosis of 108 cases. Pathol Res Pract. 1986;181:684-92.
20. Thomas S, Bhake A. Cytodiagnosis of unusual metastases in parotid gland. J Oral Maxillofac Pathol. 2021;25:171-6.
21. Cain AJ, Goodlad J, Denholm SW. Metachronous bilateral submandibular gland metastases from carcinoma of the breast. J Laryngol Otol. 2001;115:683-4.
22. Erra S, Costamagna D. Breast cancer metastatic to the submandibular gland. Case report. G Chir. 2011;32:194-8.
23. Grage TB, Lober PH. Malignant tumors of the major salivary glands. Surgery. 1962;52:284-94.
24. Meyers AD, Olshock R. Metastasis to the submaxillary gland from the breast � a case report and literature review. J Otolaryngol. 1981;10:278�82.
25. Rosti G, Callea A, Merendi R, Beccati D, Tienghi A, Turci D, et al. Metastases to the submaxillary gland from breast cancer: case report. Tumori. 1987;73:413-6.
26. Solomon MP, Rosen Y, Gardner B. Metastatic malignancy in the submandibular gland. Oral Surg Oral Med Oral Pathol. 1975;39:469�73.
27. Vessecchia G, Di Palma S, Giardini R. Submandibular gland metastasis of breast carcinoma: a case report and review of the literature. Virchows Arch. 1995;427:349-51.
28. Leite C, Dias N, Oliveira D, Pinto RM, Vaz FC. Metaplastic breast cancer with chondroid differentiation�case report and literature review. J Surg Case Rep. 2021;2021:rjab113.
29. Gortman A, Aherne NJ, Westhuyzen J, Amalaseelan JV, Dwyer PM, Hoffmann M, et al. Metaplastic carcinoma of the breast: Clinicopathological features and treatment outcomes with long-term follow up. Mol Clin Oncol. 2021;15:178.
30. Chen R, Qarmali M, Siegal GP, Wei S. Receptor conversion in metastatic breast cancer: analysis of 390 cases from a single institution. Mod Pathol. 2020;33:2499-506.
31. Grinda T, Joyon N, Lusque A, Lef�vre S, Arnould L, Penault-Llorca F, et al. Phenotypic discordance between primary and metastatic breast cancer in the large-scale real-life multicenter French ESME cohort. NPJ Breast Cancer. 2021;7:41.
32. Bertucci F, Ng CKY, Patsouris A, Droin N, Piscuoglio S, Carbuccia N, et al. Genomic characterization of metastatic breast cancers. Nature. 2019;569:560-4.
�
V�nia Filipa Santos AlmeidaCorreio eletr�nico:
Correio eletr�nico: vaniafi@hotmail.com
�
Conflict of interest
The authors have no conflicts of interest to declare.
�
Ethical disclosures
Protection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of data. The authors declare that they have followed their work center protocols on access to patient data and for its publication.
Right to privacy and informed consent. The authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
�
CRediT authorship contribution statement
V�nia Almeida: Conceptualization, Methodology, Writing � original draft, Writing � review & editing.
Carlos Salgado: Methodology, Writing � review & editing.
Pedro Ferraz: Methodology.
Lina Carvalho: Methodology, Writing � review & editing.
�
1646-2890/� 2023 Sociedade Portuguesa de Estomatologia e Medicina Dent�ria. Published by SPEMD.
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
