
Revista Portuguesa de Estomatologia, Medicina Dentária e Cirurgia Maxilofacial
Revista Portuguesa de Estomatologia Medicina Dentária e Cirurgia Maxilofacial | 2022 | 63 (2) | 99-104
Case report
Malignant peripheral nerve sheath tumor with rhabdoid features arising in the temporal region of a patient suffering from neurofibromatosis 1 – A case report
Tumor maligno da bainha do nervo periférico com características rabdoides surgindo na região temporal de um paciente com neurofibromatose 1 – Caso clínico
a Oral & Maxillofacial Pathology Department, Faculty of Dentistry, Cairo University, Egypt
b Craniomaxillofacial Program, Naser Health Institute, Cairo Egypt
c Oral Pathology Department, Faculty of Oral and Dental Medicine, Misr International University, Cairo, Egypt
Sarah Ahmed Mohamed Mahmoud - sarah.badawy@dentistry.cu.edu.eg
Article Info
Rev Port Estomatol Med Dent Cir Maxilofac
Volume - 63
Issue - 2
Case report
Pages - 99-104
Go to Volume
Article History
Received on 28/06/2021
Accepted on 03/05/2022
Available Online on 23/06/2022
Keywords
Case Report
�
Malignant peripheral nerve sheath tumor with rhabdoid features arising in the temporal region of a patient suffering from neurofibromatosis 1 - A case report
Tumor maligno da bainha do nervo perif�rico com caracter�sticas rabdoides surgindo na regi�o temporal de um paciente com neurofibromatose 1 - Caso cl�nico
�
Hatem Wael Amer1 0000-0003-3560-5918
Hamed Abdelwahab Shaheen2 0000-0002-0230-8952
Madiha Nabil Ashoub3 0000-0002-4844-5215
Sarah Ahmed Mohamed Mahmoud1,* 0000-0002-6614-6796
1 Oral & Maxillofacial Pathology Department, Faculty of Dentistry, Cairo University, Cairo, Egypt
2 Craniomaxillofacial Program, Naser Health Institute, Cairo, Egypt
3 Oral Pathology Department, Faculty of Oral and Dental Medicine, Misr International University, Cairo, Egypt
�
�
Article history:
Received 28 June 2021
Accepted 3 May 2022
Available online 22 June 2022
�
Abstract
The malignant peripheral nerve sheath tumor is one of the rare sarcomas that have a challenging diagnosis. It occurs independently with neurofibromatosis type 1. Some studies referred that malignant peripheral nerve sheath tumor attains a worse prognosis when it occurs in the head and neck region. In the reported case, a male patient with neurofibromatosis type 1 manifestations presented with a large mass in the temporal region. Based on the clinical history, histopathology, and immunohistochemical findings, the lesion was diagnosed as a low-grade malignant peripheral nerve sheath tumor with rhabdoid features. Surgical resection followed by intensity-modulated radiotherapy was the treatment of choice.
Keywords: Head; Malignant peripheral nerve sheath tumor; Malignant triton tumor; Neurofibromatosis 1
�
Resumo
O tumor maligno da bainha do nervo perif�rico � um dos raros sarcomas que t�m um diagn�stico desafiador. Ocorre independentemente com a neurofibromatose tipo 1. Alguns estudos referem que o tumor maligno da bainha do nervo perif�rico apresenta pior progn�stico quando ocorre na regi�o da cabe�a e pesco�o. No caso relatado, um paciente do sexo masculino com manifesta��es de neurofibromatose tipo 1 apresentou uma grande massa na regi�o temporal. Com base na hist�ria, histopatologia e achados imuno-histoqu�micos, a les�o foi diagnosticada como um tumor maligno de bainha de nervo perif�rico de baixo grau com caracter�sticas rabdoides. A ressec��o cir�rgica seguida de radioterapia de intensidade modulada foi o tratamento de escolha.
Palavras-chave: Cabe�a; Tumor maligno da bainha do nervo; Perif�rico; Tumor de Trit�o maligno; Neurofibromatose 1
�
Introduction
Neurofibromatosis 1 (NF1) is the most common type of neurofibromatosis (NF).1 The earliest clinical manifestations of NF1 are caf�-au-lait pigmentations, axillary freckling, and Lisch nodules. Other manifestations include skeletal deformities and optic glioma. Neurofibroma and plexiform neurofibroma tend to manifest in early adolescence.2
The malignant peripheral nerve sheath tumor (MPNST) is a rare type of sarcoma that accounts for 2-6% of all head and neck sarcomas.1 MPNST can be associated with NF1, which occurs in 50% of MPNST cases. In turn, MPNST is reported to develop in 8-12% of NF1 cases, mainly arising from a pre-existing plexiform neurofibroma.3 Le Guellec et al.4 reported that MPNST cases related to NF1 tend to occur in younger age groups and present as more rapidly growing masses than sporadic cases. Cai et al.5 evaluated the prognosis of MPNST cases in the English literature and found that cases associated with NF1 and cases in the head and neck region had a poorer prognosis and lower overall survival rate. This article reports a case of low-grade MPNST with rhabdoid features arising from a plexiform neurofibroma in the temporal region of a patient with NF1. It analyzes the management and prognosis of the case.
Case report
A 20-year-old male patient presented with a large well-circumscribed swelling that extended across the right temporal, zygomatic, and parotid regions, causing downward displacement of the auricle (Figures 1 and 2). The previous year, the patient had suffered from a slow-growing mass on the same site of the current lesion, which was diagnosed as a plexiform neurofibroma. The patient was diagnosed with NF1 in early childhood. He had numerous caf�-au-lait pigmentations with smooth borders on his arms, back, chest, and neck (Figures 1 and 2). Moreover, he had multiple nodules on his neck, back, and arms, diagnosed as neurofibromas whenever excised.
�
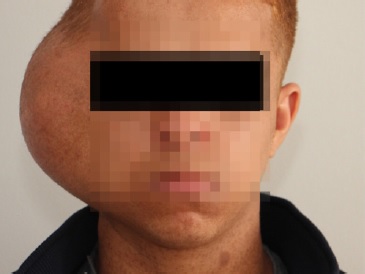
Figure 1. Frontal view of the lesion showing facial asymmetry.
�
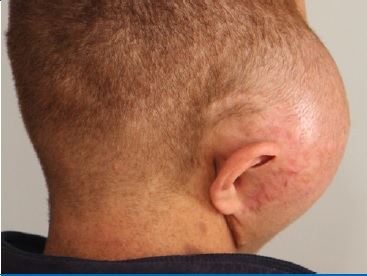
Figure 2. Back view of the lesion showing the downward displacement of the auricle.
�
There was no family history of NF1. MRI examination revealed a well-defined subcutaneous mass involving the right side of the face and temple, with 12.5 x 8.8 x 5.8 cm (craniocaudal/ anteroposterior/ transverse), that showed a heterogeneous signal, being hypointense in T1W sequence and hyperintense in T2W. The mass caused a lower displacement of the right earlobe overlaying the ramus of the mandible and located superficial to the parotid gland. Furthermore, the lesion showed a deep extension into the right parapharyngeal space (Figure 3). The patient performed a PET scan that revealed he was free of any metastasis.
�
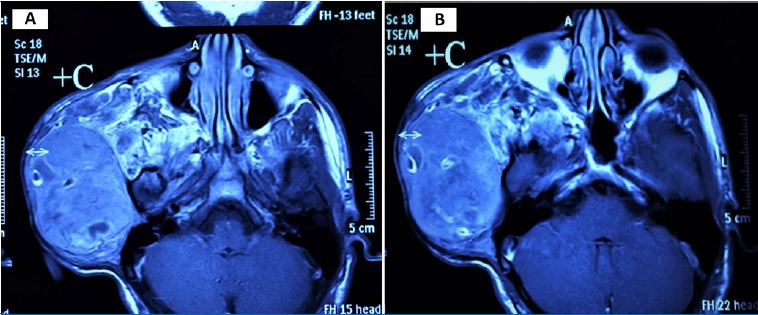
Figure 3. MRI scan of the lesion
�
The lesion was approached through a modified Blair incision with temporal and cervical extensions (Figure 4). The temporal part of the lesion, which was removed through extracapsular dissection, was displacing the facial tissues rather than infiltrating and its deep surface was against the temporal bon� (Figure 5). The parapharyngeal part was approached through the cervical extension of the incision and was removed by blunt finger dissection.
�
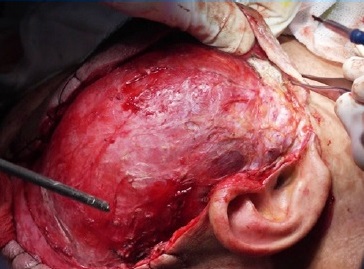
Figure 4. Intraoperative image: modified Blair incision with temporal and cervical extensions.
�
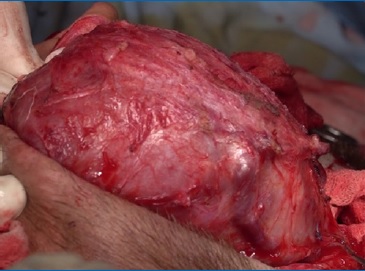
Figure 5. Intraoperative image: the temporal part of the lesion was displacing the facial tissues rather than infiltrating them.
�
The excised lesion was sent to the pathology lab, which revealed hypercellular interlacing fascicles of spindle cells with hyperchromatic wavy thin nuclei. Cellular atypia and increased mitosis (≥5 mitosis/ 10 HPF) were evident in all fields (Figure 6). Some fields revealed ovoid cells with eosinophilic cytoplasm and others with vacuolated cytoplasm, giving the appearance of �spider cells� (Figures 6 C and D). Little necrosis and hemorrhage were detected. Immunohistochemical staining with S100 protein revealed patchy nuclear and cytoplasmic positivity (Figure 7).
�
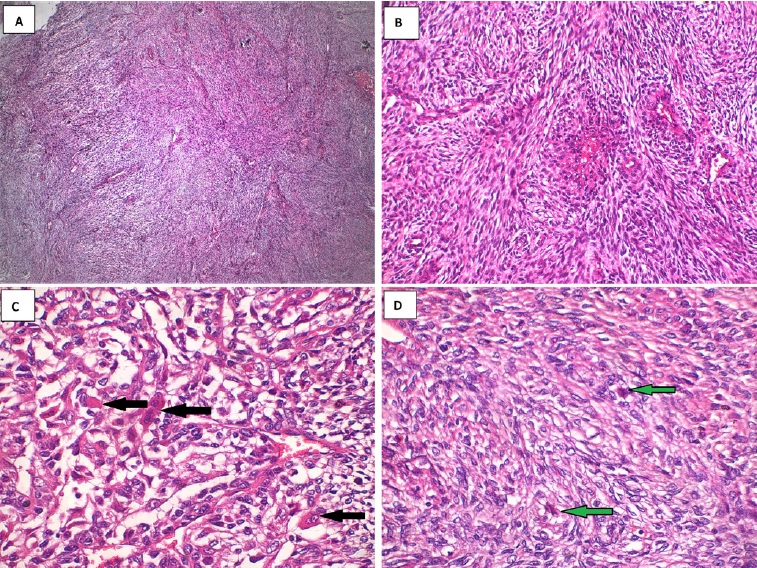
Figure 6. Histopathological findings: A. Marbled appearance due to alternating hypocellular and hypercellular areas; B. Perivascular accentuation and evident herringbone growth pattern; C. Increased mitosis and rhabdoid cells with eosinophilic cytoplasm (black arrows); D. Rhabdoid cells with vacuolated cytoplasm (�spider cells� green arrows).
�
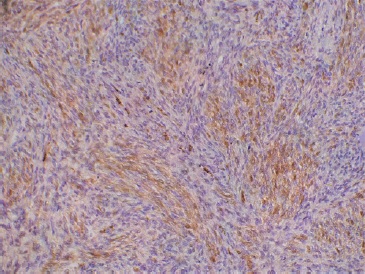
Figure 7. Immunohistochemical staining showing focal positivity of S100.
�
Based on the histopathological and immunohistochemical staining, along with the history of the lesion, the lesion was diagnosed as a low-grade MPNST. Two weeks after the operation, the patient came for follow-up (Figure 8), and the MRI scan revealed only a considerable amount of post-surgical edema (Figure 9). The patient started high precision intensity- modulated radiotherapy (IMRT) up to 60 Gy. The patient has been under ongoing close follow-up for the last 6 months. He did not report complaints during the check-ups. The exact timeline of the case can be seen in Table 1.
�
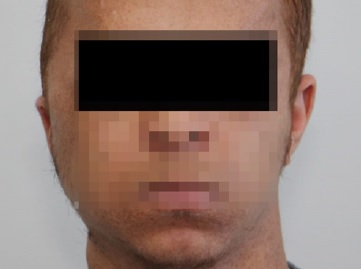
Figure 8. Clinical findings two weeks after the operation.
�
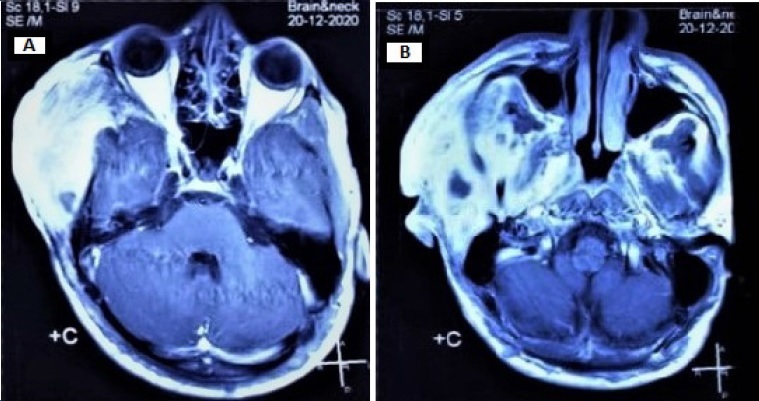
Figure 9. Radiographic findings two weeks after the operation.
�
Table 1. Timeline of the case
�
�
Discussion and conclusions
Generally, NF1 is considered one of the most prevalent autosomal dominant genetic conditions affecting human beings.6 The pathogenesis of NF1 is based on the loss of function mutation that occurs in the neurofibromin 1 gene, located on chromosome 17q11.2 Normally, neurofibromin serves as a tumor suppressor protein, responsible for activating the GTPase-activating protein, which leads to the inactivation of Ras and inhibition of cell proliferation.2 However, half of the NF1 cases result from spontaneous mutations, as in our case.2 Extra attention should be given to the plexiform variant of neurofibroma, which is regarded as a precursor for MPNST, as occurred in the present case.2, 3
Miettinen et al.6, 7 proposed nomenclature for NF1-associated lesions. The first entity, the atypical neurofibromatous neoplasm of uncertain biologic potential (ANNUBP), has at least two of the following features: nuclear atypia, hypercellularity, loss of neurofibroma architecture, and/or mitotic activity >1/ 50 HPFs and <3/ 10 HPFs. On the other hand, low-grade MPNST shows no necrosis and has a mitotic index of 3- 9/10 HPFs. In turn, high-grade MPNST is characterized by necrotic areas and a high mitotic index of >10/ 10 HPFs. According to the above-mention scheme, our case fulfilled the criteria of low-grade MPNST.
In our case, there was focal positivity for S-100 staining, although S-100 may show decreased sensitivity for MPNST.8 The current most specific marker of MPNST is the loss of H3K27me3 (trimethylated histone 3 at lysine residue 27), which has been reported to occur in 61% of MPNST cases, including those associated with NF1. It is also useful in differentiating between ANNUBP and MPNST as it is not lost in ANNUBP.8 Since our case showed positivity to the S-100 protein and was associated with NF1, we considered there was no need to do the H3K27me3 staining.
In the literature, the MPNST containing cells with rhabdoid features was called malignant triton tumor (MTT) and is a rare subtype of MPNST. Some authors suggested that the origin of the neural cells and rhabdoid cells in MPNST is undifferentiated neural crest cells.9 Based on the previously reported cases, MTT was postulated as having a highly aggressive clinical behavior and a low 5-year survival rate.9
In general, when MPNST is compared to other soft-tissue sarcomas, it is considered to have a worse prognosis, with up to 65% local recurrence rate and up to 68% distant metastasis.
Several prognostic factors such as tumor size, tumor site, histopathological grade, and involvement of surgical margins have been suggested, but no consensus has been reached.10
There is a conflict in the literature on whether MPNST�s association with NF1 and occurrence in the head and neck region lead to a worse prognosis.5 In our case, the close follow-up of the NF1 diagnosed patient led to the early diagnosis of the malignancy. Moreover, although our case was in the temporal region, it revealed a low-grade lesion, contradicting the opinion of the worse prognosis of head and neck lesions. Due to the irresponsive feature of the tumor to definitive chemoradiotherapy, the treatment of choice is surgical resection with wide safety margins, along with adjuvant radiation therapy for large tumors.5
We conclude that the prognosis of MPNST or MTT is multifactorial and cannot be determined by just one prognostic factor. Furthermore, the association of MPNST with NF1 may lead to an early diagnosis of the lesion due to the close follow-up.
�
References
1. Lee S, Lee C, Kim JK, Nam W. An unusual presentation of intraosseous malignant peripheral nerve sheath tumour of mandible. Dentomaxillofac Radiol. 2019;48:20180341.
2. Hirbe AC, Gutmann DH. Neurofibromatosis type 1: A multidisciplinary approach to care. Lancet Neurol. 2014;13:834-43.
3. Jett K, Friedman JM. Clinical and genetic aspects of neurofibromatosis 1. Genet Med. 2010;12:1-11.
4. Le Guellec S, Decouvelaere AV, Filleron T, Valo I, Charon-Barra C, Robin YM, et al. Malignant peripheral nerve sheath tumor is a challenging diagnosis: A systematic pathology review, immunohistochemistry, and molecular analysis in 160 patients from the French sarcoma group database. Am J Surg Pathol. 2016;40:896-908.
5. Cai Z, Tang X, Liang H, Yang R, Yan T, Guo W. Prognosis and risk factors for malignant peripheral nerve sheath tumor: a systematic review and meta-analysis. World J Surg Oncol. 2020;18:1-12.
6. Sayah C, Benmahmoud M. Neurofibromatosis type 1 (NF1): Case report and review of literature. J Child Dev Disord. 2016;2:1-4.
7. Miettinen MM, Antonescu CR, Fletcher CDM, Kim A, Lazar AJ, Quezado MM, et al., Histopathologic evaluation of atypical neurofibromatous tumors and their transformation into malignant peripheral nerve sheath tumor in patients with neurofibromatosis 1-a consensus overview. Hum Pathol. 2017;67:1-10.
8. Mustapar N, Zawawi MSF, Tuan Sharif SE. The value of H3K27me3 immunohistochemistry in differentiating malignant peripheral nerve sheath tumour with its histologic mimickers. Asian Pacific J Cancer Prev. 2020;21:699-705.
9. Chaudhry I, Algazal T, Cheema A, et al. Mediastinal malignant triton tumor: A rare case series and review of literature. Int J Surg Case Rep. 2019,62:115-9.
10. Czarnecka AM, Sobczuk P, Zdzienicki M, Spałek M, Rutkowski P. Malignant peripheral nerve sheath tumour (MPNST). Oncol Clin Pract. 2018;14:364-76.
�
�Sarah Ahmed Mohamed Mahmoud
Correio eletr�nico: sarah.badawy@dentistry.cu.edu.eg
�
Conflict of interest
The authors have no conflicts of interest to declare.
�
Ethical disclosures
Protection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of data. The authors declare that they have followed their work center protocols on access to patient data and for its publication.
Right to privacy and informed consent. The authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
�
CRediT authorship contribution statement
Hatem W. Amer: Conceptualization, Funding acquisition, Investigation, Resources, Supervision, Visualization, Writing - review & editing.
Hamed A. Shaheen: Funding acquisition, Investigation, Methodology, Project administration, Resources, Validation, Visualization, Writing - original draft.
Madiha N. Ashoub: Data curation, Formal analysis, Project administration, Writing - originaldraft.
Sarah A. M. Mahmoud: Data curation, Formal analysis, Investigation, Methodology, Software, Supervision, Validation,Visualization, Writing - review & editing
�
� 2022 Sociedade Portuguesa de Estomatologia e Medicina Dent�ria. Published by SPEMD.
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
