
Revista Portuguesa de Estomatologia, Medicina Dentária e Cirurgia Maxilofacial
SPEMD | 2019 | 60 (4) | 205-209
Case report
Trichilemmoma of the perioral area – Case report
Triquilemoma da região perioral – Caso clínico
a Instituto de Implantologia, Lisbon, Portugal
b Faculdade de Medicina Dentária, Universidade de Lisboa, Lisbon, Portugal
c Unidade de Diagnóstico Histológico e Citológico, Lisbon, Portugal
d LIBPhys-FCT UID/FIS/04559/2013
Article Info
Rev Port Estomatol Med Dent Cir Maxilofac
Volume - 60
Issue - 4
Case report
Pages - 205-209
Go to Volume
Article History
Received on 28/09/2019
Accepted on 08/11/2019
Available Online on 20/12/2019
Keywords
Case report
�
Trichilemmoma of the perioral area � Case report
Triquilemoma da regi�o perioral � Caso cl�nico
�
Filipe Freitas a,b,*, Saudade Andr�c, Andr� Pereiraa, Andr� Moreiraa, Duarte Marquesa,b,d, Jo�o Caram�s a,b,d
a Instituto de Implantologia, Lisbon, Portugal
b Faculdade de Medicina Dentaria, Universidade de Lisboa, Lisbon, Portugal
c Unidade de Diagnostico Histologico e Citologico, Lisbon, Portugal
d LIBPhys-FCT UID/FIS/04559/2013
�
�
http://doi.org/10.24873/j.rpemd.2019.12.686
�
Abstract
The trichilemmoma is a benign cutaneous epithelial tumor that results from a hamartomatous proliferation of cells from the hair follicle. The multiplication of glycogen-rich clear cells causes a papular or nodular lesion of the skin. Trichilemmomas may be solitary or multiple (associated with Cowden�s syndrome). The desmoplastic trichilemmoma is a histologic variant that resembles invasive carcinomas such as trichilemmal carcinoma, basal-cell carcinoma or squamous-cell carcinoma. A 68-year-old male presented with an asymptomatic ulcerated papule in his perioral area, after being referred to an oral surgeon by his dental hygienist. The lesion was excised and the histopathologic examination led to the diagnosis of conventional trichilemmoma. At the 1-year follow-up, the patient was free from recurrence.
Keywords: Cowden�s syndrome, Desmoplastic trichilemmoma,Trichilemmal carcinoma,Trichilemmoma
�
Resumo
O triquilemoma � um tumor benigno dos anexos cut�neos, traduzindo uma prolifera��o hamartomatosa das c�lulas dos fol�culos pilosos. Resulta da multiplica��o de c�lulas claras ricas em glicog�nio e origina, habitualmente, uma les�o exof�tica papular ou nodular. Podem surgir les�es �nicas ou m�ltiplas, associadas, neste caso, � s�ndrome de Cowden. O triquilemoma desmopl�sico � uma variante histol�gica que pode simular um carcinoma invasivo, como os carcinomas triquilemal, basocelular ou pavimentocelular. Ser� descrito um caso cl�nico de um doente do sexo masculino, com 68 anos, que foi referenciado para um cirurgi�o oral pelo seu higienista por apresentar uma p�pula ulcerada, assintom�tica, na regi�o perioral.
A les�o foi alvo de bi�psia excisional, tendo o exame anatomopatol�gico revelado tratar-se de um triquilemoma convencional. O controlo peri�dico do doente foi realizado um ano depois, n�o tendo sido observada qualquer recidiva da les�o.
Palavras-chave: S�ndrome de Cowden,Triquilemoma desmopl�sico,Carcinoma triquilemal,Triquilemoma
�
Introduction
Trichilemmoma was first described in 1962 by Headington and French, in their treatise on the histogenesis and classification of primary neoplasms of the hair follicle, as a benign solid cutaneous epithelial tumor composed of clear cells, with differentiation toward the outer hair root sheath.1 It is a hamartomatous proliferation arising from glycogen-rich epithelial cells.2
Trichilemmomas can be solitary or multiple. The single presentation is usually found on the face of adults or the elderly, as a small papule or nodule, similar in color to the underlying skin or slightly erythematous, with a smooth or warty surface. It sometimes exhibits central ulceration as well as keratinization of the epidermal surface, which may be sufficient for the formation of pearls or a cutaneous horn.3,4 It may also involve other locations, such as the neck, scalp, thoracic skin or vulva.2,3
It presents a slow, progressive growth, and the evolution over time can vary from months to years, with its diameter rarely exceeding 1 cm.5
The presence of multiple facial trichilemmomas is associated with Cowden syndrome, originally described by Costello in 1940 and named by Lloyd and Dennis in 1963.6 In 1972, Wear and colleagues described several distinctive mucocutaneous features, such as hamartomatous lesions of ectodermal, mesodermal and ectodermal origin, and proposed the designation �multiple hamartoma syndrome.�7 Later, in 1977, Brownstein and colleagues suggested cutaneous trichilemmomas as a specific marker of this condition.8,9 The diagnostic criteria were reviewed at an international consensus meeting that established that the main pathognomonic lesions consist of multiple cutaneous papules, papillomatous alterations of the oral mucosa corresponding to fibromas, as well as palmar or plantar acral keratosis.10
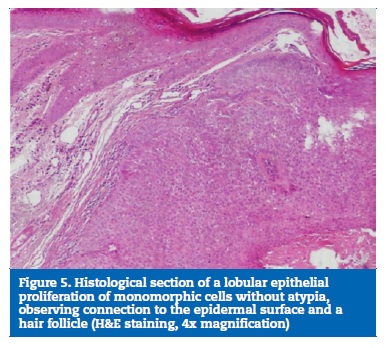
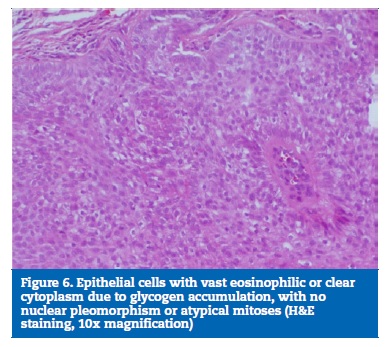
Histopathologically, trichilemmomas are small and circumscribed lobular or multilobular proliferations, consisting of clear cells from the root of the hair follicle, usually with a large connection to the epidermal surface.2,3,5 The cells� pale appearance is due to the accumulation of glycogen inside them and can be evidenced by periodic acid-Schiff (PAS) staining.5 A papillomatous surface is common, reflecting the presence of verrucous hyperplasia with hypergranulosis.5 Peripherally, each lobe also has a small and compact layer of columnar cells arranged in palisade, formed by keratinocytes, usually with a thick adjacent basement membrane.3,5 As a sign of their differentiation into the hair follicle�s outer root sheath, trichilemmomas express CD34, a feature that has been used to distinguish them from other epithelial tumors through immunohistochemical analysis.11-13
In 1990, Hunt and colleagues described a rare histological variant of trichilemmoma that contains areas of narrow irregular cords of epithelial cells trapped in a dense eosinophilic hyaline stroma resembling an invasive carcinoma and called it desmoplastic trichilemmoma.14 It is predominant in women after the fifth decade of life.15,16
Another relevant condition is the trichilemmal carcinoma, initially known as clear cell carcinoma of the skin, which is a malignant neoplasm that combines trichilemmal keratinization with atypical changes such as intense mitotic activity, reticular dermis invasion and ulceration.17-21
The differential diagnosis of trichilemmoma includes other epidermal or hair follicle tumors, namely trichofolliculoma, fibroma, inverted follicular keratosis, seborrheic keratosis, intradermal melanocytic nevus and keratoacanthoma.22-25
Lesions associated with human papillomavirus such as wart vulgaris or squamous papilloma should also be taken into account.26 Trichilemmal carcinoma, basal-cell carcinoma and squamous-cell carcinoma are malignant entities that should integrate the differential diagnosis of desmoplastic trichilemmoma.14,26
The treatment of trichilemmomas consists of surgical excision, being the recurrence rare, which contributes to a good prognosis.1,2,27
The aim of this paper is to describe a clinical case of a trichilemmoma in the perioral region, addressing the clinical and histological characteristics that allowed its diagnosis, as well as review the treatment suggested in the literature for this pathology, in order to contribute to a greater knowledge of this rare condition.
Case report
A 68-year-old male patient presented with an asymptomatic lesion in his right lip commissure region after referral by his oral hygienist for an oral medicine appointment. No relevant medical history and no smoking or alcoholism were stated.
The patient was under treatment with simvastatin to control hypercholesterolemia and pregabalin due to cervical spinal canal stenosis.
At clinical examination, a sessile exophytic cutaneous lesion with papular appearance, 5 mm in diameter with central ulceration was identified. The patient reported a slow growth, with more than 12 months of evolution (Figures 1 and 2).
�
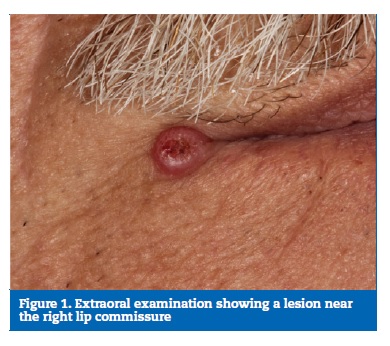
�
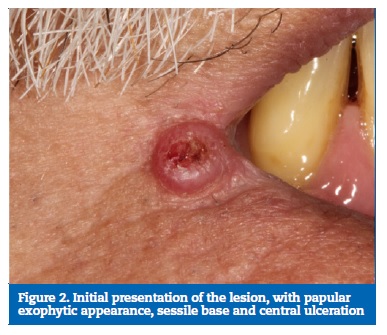
�
The clinical characteristics observed were suggestive of benign proliferation, and the lesion was compatible with a keratoacanthoma. The differential diagnosis included intradermal nevus, wart vulgaris and trichilemmoma.
Surgical excision of the lesion was proposed. An excisional biopsy was performed under local anesthesia with 2% lidocaine perilesional infiltration with 1: 80,000 epinephrine (Xilonibsa, INIBSA, Portugal). An elliptical incision was made with scalpel blade 15, followed by suturing of the wound with four single stitches using 5.0-diameter polypropylene monofilament yarn (Hu-Friedy, USA) (Figure 3).
�
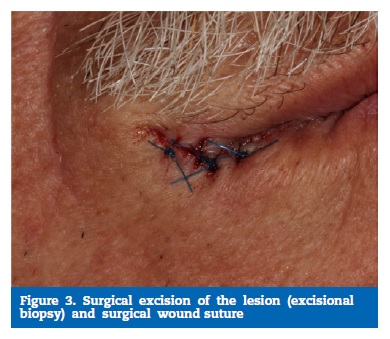
�
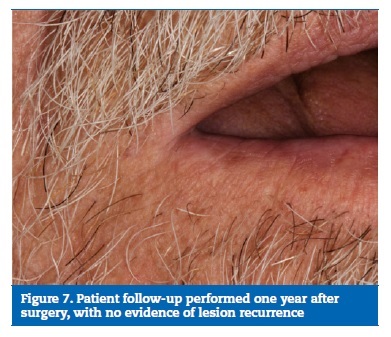
Acetaminophen was prescribed for pain relief, and sutures were removed eight days after surgery (Figure 4). The surgical specimen was fixed in buffered formalin and sent for anatomopathological examination, which revealed a fully excised trichilemmoma (Figures 5 and 6). One year later, the patient�s follow-up revealed no recurrence of the lesion (Figure 7).
�
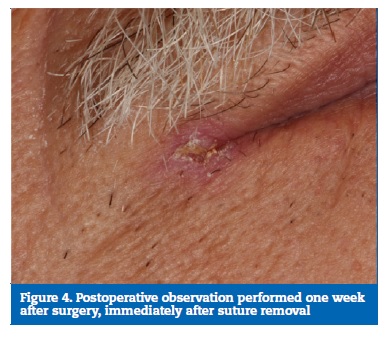
�
�
�
�
Discussion and conclusions
The cells that originate trichilemmoma appear to be located in a superficial area of the hair follicle, just below the basement membrane, at the level of the sebaceous gland.
There is a lobular proliferation of small uniform cells to the dermis from the epidermis, and the presence of clear cells containing glycogen is the most notable alteration.1 - 4
The relationship between trichilemmoma and common wart is controversial, and trichilemmomas are actually often clinically diagnosed as warts. On the other hand, areas histologically similar to trichilemmomas are often found in warts.
Some authors, therefore, believe that trichilemmomas may correspond to mere mature warts, with some period of evolution, that have suffered trichilemmal differentiation.9 However, several studies have failed to detect the human papillomavirus in trichilemmomas.28, 29
Solitary trichilemmomas should be surgically excised, with a peripheral margin of 2 mm and reaching the fullest depth extent of the tumor.4, 30, 31 The differential diagnosis of conventional or desmoplastic trichilemmomas should include other benign or malignant tumors of the skin or cutaneous appendages. However, all these entities have distinctive architectural patterns and, unlike trichilemmomas, do not have clear cells or a thick basement membrane.3 The recognition of the desmoplastic variant, which mimics an invasive carcinoma, is particularly important.14 Therefore, the correct distinction between desmoplastic trichilemmoma and the various types of carcinoma, namely trichilemmal carcinoma, squamous-cell carcinoma and sclerosing basal-cell carcinoma, is fundamental. The circumscription of the lesion, the identification of chords and small epithelial cell nests fused with a desmoplastic stroma in the central tumor area, the expression of CD34 and the absence of obvious squamous or basaloid differentiation favor the diagnosis of desmoplastic trichilemmoma, excluding the presence of malignancy.3, 32
Multiple facial trichilemmomas are observed in about 99% of Cowden�s syndrome patients, whose transmission is autosomal dominant, despite the incomplete penetrance and variable expression.33 This multiple hamartoma syndrome results from a mutation in a tumor suppressor gene located on chromosome 1034 that was simultaneously identified and named by three distinct research groups as PTEN, MMAC1 and TEP1.35 - 37
Patients with this condition are more likely to develop malignant neoplasms, such as breast, thyroid and gastrointestinal cancer.10, 38, 39 Oral manifestations are one of the main criteria for the diagnosis of this condition and, therefore, oral health professionals should be aware of them.10
�
References
1. Headington JT, French AJ. Primary neoplasms of the hair follicle. Histogenesis and classification. Arch Dermatol. 1962;86:430-41.
2. Brownstein MH, Shapiro L. Trichilemmoma. Analysis of 40 new cases. Arch Dermatol. 1973;107:866-9.
3. Herraiz M, Martin-Fragueiro LM, Tardio JC. Trichilemmoma arising in the nasal vestibule: report of three cases with special emphasis on the differential diagnosis. Head and Neck Pathol. 2012;6:492-5.
4. Sharma R, Sirohi D, Sengupta P, Sinha R, Menon PS. Desmoplastic trichilemmoma of the facial region mimicking invasive carcinoma. J Maxillofac Oral Surg. 2011;10:71-3.
5. Sano DT, Yang JJ, Tebcherani AJ, Bazzo LA. A rare clinical presentation of desmoplastic trichilemmoma mimicking invasive carcinoma. An Bras Dermatol. 2014;89:796-8.
6. Lloyd KM, Dennis M. Cowden�s disease. A possible new symptom complex with multiple system involvement. Ann Intern Med. 1963;58:136-42.
7. Weary PE, Gorlin RJ, Gentry WC, Comer JE, Greer KE. Multiple hamartoma syndrome (Cowden�s disease). Arch Dermatol. 1972;106:682-90.
8. Brownstein MH, Mehregan AH, Bikowski JB. Trichilemmomas in Cowden�s disease. JAMA. 1977;238:26.9. Brownstein MH, Mehregan AH, Bikowski JB, Lupulescu A, Patterson JC. The dermatopathology of Cowden�s syndrome. Br J Dermatol. 1979;100:667-73.
10. Pilarski R, Eng C. Will the real Cowden syndrome please stand up (again)? Expanding mutational and clinical spectra of the PTEN hamartoma tumour syndrome. J Med Genet. 2004;41: 323-6.
11. Poblet E, Jimenez-Acosta F, Rocamora A. QBEND/10 (anti-CD34 antibody) in external root sheath cells and follicular tumors. J Cutan Pathol. 1994;21:224-8.
12. Illueca C, Monteagudo R, Revert A, Llombart-Bosch A. Diagnostic value of CD34 immunostaining in desmoplastic trichilemmoma. J Cutan Pathol. 1998;25:435-9.
13. Tardio JC. CD34-reactive tumors of the skin. An update review of an ever- growing list of lesions. J Cutan Pathol. 2009;36:89-102.
14. Hunt SJ, Kilzer B, Santa Cruz DJ. Desmoplastic trichilemmoma: histologic variant resembling invasive carcinoma. J Cutan Pathol. 1990;17:45-52.
15. Schweiger E, Spann CT, Weinberg JM, Ross B. A Case of Desmoplastic Trichilemmoma of the Lip Treated with Mohs Surgery. Dermatol Surg. 2004;30:1062-4.
16. Topping NC, Chakrabarty A, Edrich C, Henderson T. Desmoplastic trichilemmoma of the upper eyelid. Eye (Lond). 1999;13:593-4.
17. Lee JY, Tang CK, Leung YS. Clear cell carcinoma of the skin. A tricholemmal carcinoma? J Cutan Pathol. 1989;20:31-9.
18. Swanson PE, Marrogi AJ, Williams DJ, Cherwitz DL, Wick MR. Tricholemmal carcinoma. Clinicopathologic study of 10cases. J Cutan Pathol. 1992;19:100-9.
19. Boscaino A. Tricholemmal carcinoma. A study of seven cases. J Cutan Pathol. 1992;19:94-9.
20. Reis JP, Tellechea O, Cunha MF, Baptista AP. Trichilemmal carcinoma. Review of 8 cases. J Cutan Pathol.1993; 20:44-9.
21. Wong TY, Suster S. Tricholemmal carcinoma. A clinicopathologic study of 13 cases. Am J Dermatopathol. 1994;16:463-73.
22. Park SY, Han WJ, Kim KJ, Noh KK. A case of trichofolliculoma in the nasal vestibule. Korean J Otolaryngol-Head Neck Surg. 2007;50:265-7.
23. Villaret AB, Gily B, Aga A. Inverted follicular keratosis of the nasal vestibule. Otolaryngol-Head Neck Surg. 2009;141:288-9.
24. McGlashan JA, Rees G, Bowdler DA. Solitary keratoacanthoma of the nasal vestibule. J Laringol Otol. 1991;105:306-8.
25. Buyuklu F, Aydin H, Tarhan E, Ada S, Cakmak O. Seborrheic keratosis of the nasal vestibule. Kulak Burun Bogaz Ihtis Derg. 2007;17:298-300.
26. Carr RA, Sanders DSA. Basaloid skin tumours: mimics of basal cell carcinoma. Cur Diagn Pathol. 2007;13:273-300.
27. Ackerman AB, Wade TR. Tricholemmoma. Am J Dermatopathol. 1980;2:207-24.
28. Leonardi CL, Zhu WY, Kinsey WH, et al. Trichilemmomas are not associated with human papillomavirus DNA. J Cutan Pathol. 1991;18:193-7.
29. Stierman S, Chen S, Nuovo G, et al. Detection of human papillomavirus infection in trichilemmomas and verrucae using in situ hybridization. J Cutan Pathol. 2010;37:75-80.
30. Ruhoy SM, Thomas D, Nuovo GJ. Multiple inverted follicular keratoses as a presenting sign of Cowden�s syndrome: case report with human papillomavirus studies. J Am Acad Dermatol. 2004;51:411-5.
31. Krishnakumar S, RavindraMohan E, Kalpana B, Das D, Biswas J. Eccrine duct carcinoma of the eyelid mimicking meibomian carcinoma: clinicopathological study of a case. Surv Ophthalmol. 2003;48:439-46.
32. Sanders DSA, Carr RA. The use of immunohistochemistry in the differential diagnosis of common epithelial tumoursof the skin. Curr Diagn Pathol. 2007;13:237-51.
33. Nuss DD, Aeling JL, Clemons DE, Weber WN. Multiple hamartomas syndrome (Cowden�s disease). Arch Dermatol 1978;114:743-6.
34 Nelen MR, Padberg GW, Peeters EAJ, Lin AY, van den Helm B, Frants RR, et al. Localization of the gene for Cowden disease to chromosome 10q22-23. Nat Genet. 1996;13:114-6.
35. Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 1997;275:1943-7.
36. Steck PA, Pershouse MA, Jasser SA, Yung WK, Lin H, Ligon AH, et al. Identification of a candidate tumour suppressor gene. MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet 1997;15:356-62.
37. Li DM, Sun H. TEP1, encoded by a candidate tumor suppressor locus, is a novel protein tyrosine phosphatase regulated by transforming growth factor beta. Cancer Res 1997;57:2124-9.
38. Brownstein MH, Wolf M, Bikowski JB. Cowden�s disease: a cutaneous marker of breast cancer. Cancer. 1978;41:2393-8.
39. Carlson JG, Nivatvongs S, Snover DC. Colorectal polyps in Cowden�s disease (multiple hamartoma syndrome). Am J Surg Pathol.1984;8:763-70.
�
Filipe Freitas
E-mail address: filipefreitas@institutoimplantologia.com
�
Ethical disclosures
Protection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of data. The authors declare that they have followed the protocols of their work center on access to patient data and for its publication.
Right to privacy and informed consent. The authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
�
Conflict of interest
The authors have no conflicts of interest to declare.
�
Article history:
Received 28 September 2019
Accepted 8 November 2019
Available online 20 December 2019
