
Revista Portuguesa de Estomatologia, Medicina Dentária e Cirurgia Maxilofacial
SPEMD - Rev Port Estomatol Med Dent Cir Maxilofac | 2019 | 60 (2) | 79-84
Case report
Ectomesenchymal chondromyxoid tumor with a significant proliferative index: A case report
Tumor condromixoide ectomesenquimal com um índice proliferativo muito significante: Relato de um caso
a Dentistry and Health Postgraduate Program, School of Dentistry, Federal University of Bahia, Salvador-Bahia, Brazil
b Department of Oral Pathology and Medicine, School of Dentistry, University of Chile. Santiago, Chile
c Laboratory of ImagePat, Salvador-Bahia, Brazil
d Department of Health I, School of Dentistry, State University of Southwestern Bahia. Jequié-Bahia, Brazil
e Laboratory of Oral Surgical Pathology, School of Dentistry, Federal University of Bahia. Salvador-Bahia, Brazil
Jean Nunes dos Santos - jeanunes@ufba.br
Article Info
Rev Port Estomatol Med Dent Cir Maxilofac
Volume - 60
Issue - 2
Case report
Pages - 79-84
Go to Volume
Article History
Received on 28/01/2019
Accepted on 21/05/2019
Available Online on 18/06/2019
Keywords
Case report
�
Ectomesenchymal chondromyxoid tumor with a significant proliferative index: A case report
Tumor condromixoide ectomesenquimal com um �ndice proliferativo muito significante: Relato de um caso
�
Daniela Adorno-Fariasa,b, Paulo Roberto Athanazioc, Adna Barros Ismerimd, Ernesto Santos Sousa Netoa, Eliabe Almeida dos Santose, Jean Nunes dos Santose,*
aDentistry and Health Postgraduate Program, School of Dentistry, Federal University of Bahia, Salvador-Bahia, Brazil
b Department of Oral Pathology and Medicine, School of Dentistry, University of Chile. Santiago, Chile
cLaboratory of ImagePat, Salvador-Bahia, Brazil
dDepartment of Health I, School of Dentistry, State University of Southwestern Bahia. Jequi�-Bahia, Brazil
eLaboratory of Oral Surgical Pathology, School of Dentistry, Federal University of Bahia. Salvador-Bahia, Brazil�
�
http://doi.org/10.24873/j.rpemd.2019.05.447�
Abstract
An ectomesenchymal chondromyxoid tumor (ECT) is a rare intraoral tumor that should be included in the differential diagnosis of other mesenchymal tumors, particularly when located in the tongue. This study reports a new case of a 34-year-old female patient with a nodular lesion on the tongue dorsum. Its clinical diagnosis was irritative fibroma. The lesion was surgically removed, and, after 2 years of follow-up, no recurrence was observed. A detailed description of the histomorphological and immunohistochemical features of the ECT was made, highlighting the high Ki-67 proliferative index observed.
Keywords: Case report,Ectomesenchymal chondromyxoid tumor, High Ki-67
�
Resumo
O tumor condromixoide ectomesenquimal (ECT) � um tumor intraoral raro, que deve ser considerado no diagn�stico diferencial de outras les�es mesenquimais de tecido mole, principalmente quando localizadas em l�ngua. Neste estudo � apresentado um novo caso de ECT, de uma paciente do sexo feminino de 34 anos de idade com uma les�o nodular em dorso de l�ngua com diagn�stico cl�nico de fibroma irritativo. A les�o foi extirpada cirurgicamente e depois de 2 anos de seguimento n�o foram observados sinais de recidiva. Ressalta-se uma descri��o detalhada das caracter�sticas histomorfol�gicas e imuno-histoqu�micas do ECT, entre as quais destacou-se o alto �ndice de prolifera��o observado para o Ki-67.
Palavras-chave: Caso cl�nico,Tumor condromixoide ectomesenquimal, Ki-67 alto
�
Introduction
An ectomesenchymal chondromyxoid tumor (ECT) is a benign mesenchymal tumor composed of cells that phenotypically resemble myoepithelial cells.1 Approximately 75 cases of ECT were published in English in the literature.2- 4 The tumor manifests as a submucosal nodule on the back of the tongue or, less frequently, at the base of the tongue and palate.2,5 The ECT is usually small, ranging from 4 to 50 mm,2 has no gender predilection, and affects patients between the first and eighth decades of life.1, 2, 6, 7 Surgical excision is indicated as treatment, and the prognosis is excellent, with a recurrence rate between 1.8 and 7%.2, 7
Histologically, the ECT is a well-delimited but not encapsulated tumor.1, 5, 8 The tumor consists of round, spindle-shaped, polygonal and oval cells2 arranged in cords and sheets or forming a reticulated pattern within a myxoid or chondromyxoid stroma.1 Atypia and multilobulated nuclei are also observed.
The immunohistochemical profile usually shows immunoreactivity to glial fibrillary acidic protein (GFAP), S100, and CD57.1, 5, 9
The aim of this study was to report a case of ECT in the oral cavity, highlighting its histopathological and immunohistochemical features.
Case Report
A 34-year-old female patient had an asymptomatic lesion on the anterior dorsum of the tongue. The nodular lesion measured 1.5 x 1.3 cm, had a sessile base, well-defined limits, normal mucosa color, smooth surface and was firm to touch.
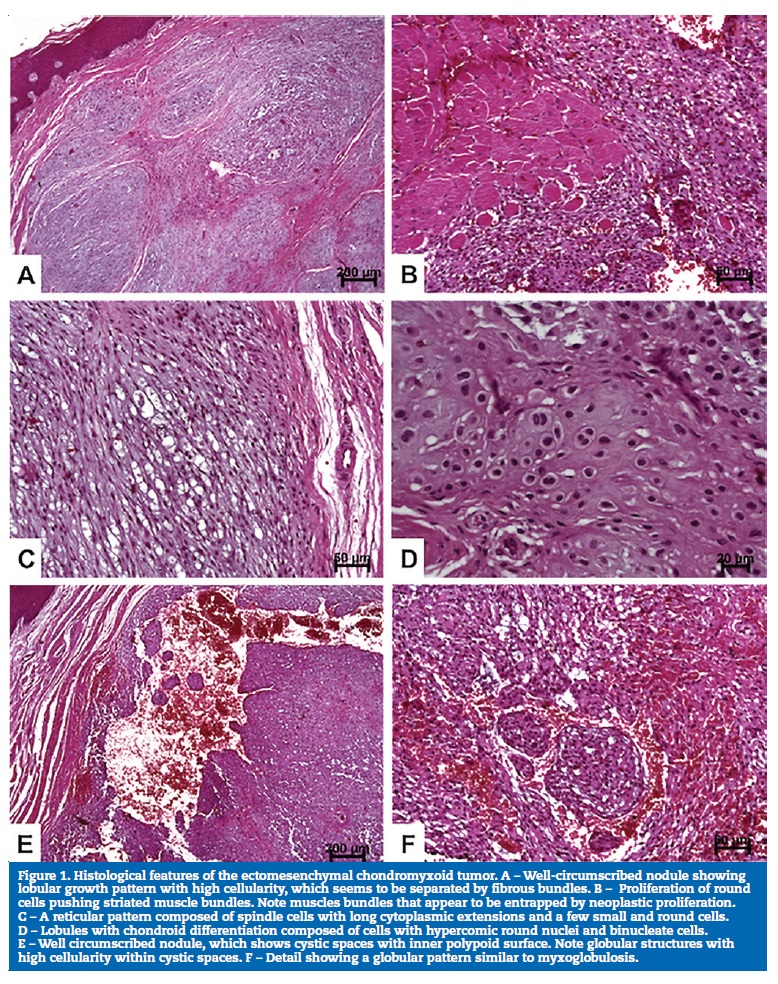
Upon a clinical diagnosis of irritation fibroma, an excisional biopsy was performed. The histopathological examination showed a well-delimited nodular lesion that pushed the striated muscle tissue, discretely surrounding it (Figures 1A and 1B). The lesion was characterized by lobules that were slightly intersected by fibrous septa (Figure 1A). The lobules exhibited frequent myxoid, chondroid and myxochondroid areas, as well as more solid areas composed of small, oval/round and hyperchromatic cells (Figures 1AC and 1D). Other areas were composed of polygonal or elongated cells with eosinophilic or vacuolar cytoplasm (Figure 1C). In addition, there were microcysts and dilated cystic spaces, sometimes with an arrangement similar to myxoglobulosis, filled with recent red blood cells (Figure 1D). Pseudoinclusions were also observed, and atypia was discrete. The histopathological features indicated the diagnosis of ECT. The deep surgical margin was focally compromised, and no neural or vascular invasion was found.
�
�
Without clinical signs of lesion remnants, the patient decided not to perform a new surgical intervention. However, the patient has still been closely monitored with serial follow-up visits. After a follow-up period of 24 months, the patient is fine, and there are no signs of lesions in the oral mucosa.
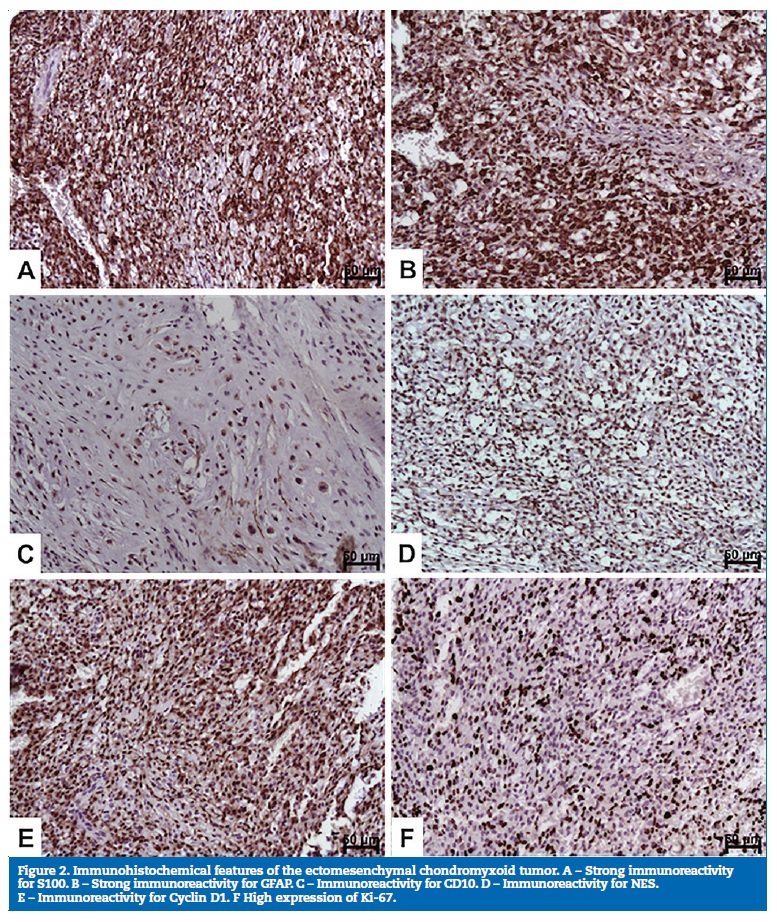
The following antibodies were used for the immunohistochemical study: S100 (Polyclonal, 1:1000; Dako, Carpinteria, CA); GFAP (Polyclonal; 1:200; Dako, Carpinteria, CA), CD10 (56C6; 1:100; Dako, Carpinteria, CA); neuron specific enolase (NES; BBS/NC/VI-H14, 1:200, Dako, Carpinteria, CA); Cyclin D1 (DCS6; 1:100; Dako, Carpinteria, CA); CD56 (123C3, 1:50; Dako, Carpinteria, CA), and Ki67 (MIB-1, 1:100; Dako, Carpinteria, CA).
Positive staining was observed for S100 (Figure 2A), GFAP (Figure 2B), CD10 (Figure 2C), NES (Figure 2D), and Cyclin D1 (Figure 2E). The Ki-67 labeling index was considered significant (60-70%) (Figure 2F), with the observation of marked immunostaining in more cellularized areas. Immunostaining was negative for CD56.
�
�
Discussion and conclusions
Although the WHO describes the ECT as a rare benign mesenchymal tumor with a myoepithelial cell-like phenotype,1, 10, 11 there is unanimous agreement that this tumor arises from neural crest cells.5,8, 12,17 The clinical features of the present case are consistent with those reported in the literature, which describe the ECT as an asymptomatic lesion that usually measures less than 2 cm in diameter, is located on the back of the tongue and affects patients at a mean age of 34 years.1 2, 5, 6, 8, 11, 12, 18, 19
Histologically, the present case meets the morphological criteria for the diagnosis of ECT.2, 4, 6, 8, 11, 15, 16, 17, 23 The tumor is characterized by a lobular growth pattern with cystic spaces and stroma with myxoid, chondroid and myxochondroid areas, and by hypercellularity � especially small cells, which seem to predominate in ECT.5 Another particular feature is neoplastic cells surrounding muscle cells,5 15 16 24 but this does not seem to represent evidence of an ECT�s aggressive behavior.22 The atypia found was discrete, but some authors speculate that these areas might be associated with inflammation secondary to stimuli or aging of the tumor.16, 18 It is important to state that we also detected globular structures, described as myxoglobulosis.24 These structures were also seen by our group in oral mucoceles.25
The immunohistochemical profile of ECT is highly variable. However, as in previous studies, the present tumor was positive for S100 and GFAP,4, 6, 7, 12, 15, 19, 23, 26, 27 demonstrating an origin from the neural crest, as well as for CD10,28 NSE20,29 and cyclin D1.4,17,28 Regarding this last protein, strong nuclear expression was detected, but no translocation of CCND1/IGH or amplification of the CCND1 gene was found.28 No CD56 staining was observed, which is in agreement with a previous study21 that reported a variable expression of this marker in its cases.
An interesting finding of the present case was the high proliferative index identified by Ki-67 staining. Conversely, a low proliferative index of this tumor has been reported in the literature.2 4, 14, 19, 26, 30 Considering the variable immunohistochemical profile of the ECT, the high proliferative index suggests the need for constant monitoring of these cases, with special attention to areas containing populations of small cells.
The main differential diagnosis of the ECT includes myoepithelioma2, 3, 5, 8, 11, 12, 15, 17, 19, 20, 23, 24, 31, 32 and pleomorphic adenoma.2, 3, 5, 7, 8, 12, 17, 20, 31 However, despite the presence of chondroid and myxoid differentiation and spindle-shaped cells, we found no plasmacytoid cells, glandular ducts, or salivary gland tissue.
Other differential diagnoses are myxoma, mucocele, oral focal mucinosis, neurofibroma, and schwannoma.2, 5, 7, 8, 12, 17, In the present case, the histological features, together with the immunohistochemical findings, helped to make this distinction since the absence of AE1/AE3 and the presence of neural markers indicated a tumor of ectomesenchymal origin.
Although rare, the ECT should be included in the differential diagnosis of nodular lesions that occur on the dorsum of the tongue. To our knowledge, this is the first case report showing a high proliferative index. Since little is known about the evolution of this tumor, the patient is monitored closely.
�
References
1. El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ. WHO Classification of Head and Neck Tumours. 4th ed. Lyon, France: IARC Press; 2017.
2. Kato MG, Erkul E, Brewer KS, Harruff EE, Nguyen SA, Day TA. Clinical features of ectomesenchymal chondromyxoid tumors: A systematic review of the literature. Oral Oncol. 2017;67:192-7.
3. Truschnegg A, Acham S, Kqiku L, Jakse N, Beham A. Ectomesenchymal chondromyxoid tumor : a comprehensive updated review of the literature and case report. Int J Oral Sci. 2018;10:1-7.
4. Almeida LY, Dominguete MHL, Dominguete PR, Ribeiro-Silva A, Teixeira LR, Le�n JE. Immune cell infiltration in Ectomesenchymal chondromyxoid tumor: Na immunohistochemical study. Oral Oncol. 2018;81:112-5.
5. Aldojain A, Jaradat J, Summersgill K, Bilodeau EA. Ectomesenchymal Chondromyxoid Tumor: A Series of Seven Cases and Review of the Literature. Head Neck Pathol. 2015;9:315-22.
6. Portnof JE, Friedman JM, Reich R, Freedman PD, Behrman DA. Oral ectomesenchymal chondromyxoid tumor: case report and literature review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2019;108e20-4.
7. Allen CM. The ectomesenchymal chondromyxoid tumor: A review. Oral Dis. 2008;14:390-5.
8. Smith BC, Ellis GL, Meis-Kindblom JM, Williams SB. The Ectomesenchymal chondromyxoid tumor of the anterior tongue. Nineteen cases of a new clinicopathologic entity. Am J Surg Pathol. 1995;19:519-30
9. Allen CM, Neville BW, Hammond HL. Adenomatoid dentinoma. Report of four cases of an unusual odontogenic lesion. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;86:313-7.
10. Woo VLK, Angiero F, Fantasia JE. Myoepithelioma of the tongue. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:581-9.
11. Angiero F. Ectomesenchymal chondromyxoid tumour of the tongue. A review of histological and immunohistochemical features. Anticancer Res. 2010;30:4685-9.
12. De Visscher JGAM, Kibbelaar RE, Van der Waal I. Ectomesenchymal chondromyxoid tumor of the anterior tongue. Report of two cases. Oral Oncol. 2003;39:83-6.
13. Yoshioka Y, Ogawa I, Tsunematsu T, Sakaue T, Yamasaki S, Fukui Y, et al. Ectomesenchymal chondromyxoid tumor of the tongue: Insights on histogenesis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115:233-40.
14. Kaplan I, Anavi Y, Calderon S. Ectomesenchymal chondromyxoid tumour of the anterior tongue. Int J Oral Maxillofac Surg. 2004;33:404-7.
15. Goveas N, Ethunandan M, Cowlishaw D, Flood TR. Ectomesenchymal chondromyxoid tumour of the tongue: Unlikely to originate from myoepithelial cells. Oral Oncol. 2006;42:1026-8.
16. Carlos R, Aguirre J, Pineda V. Ectomesenchymal chondromyxoid tumor of the tongue. Med Oral. 1999;4:361-5.
17. Cardin MJ, Fiset PO, Zeitouni AG, Caglar D. Ectomesenchymal Chondromyxoid Tumour of the Posterior Tongue. Head Neck Pathol. 2014;8:329-33.
18. Kannan R, Damm DD, White DK, Marsh W, Allen CM. Ectomesenchymal chondromyxoid tumor of the anterior tongue. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;82:417-22.
19. Palma Guzm�n JM, De Andrade BAB, Rizo VHT, Romanach MJ, Le�n JE, De Almeida OP. Ectomesenchymal chondromyxoid tumor: Histopathologic and immunohistochemical study of two cases without a chondroid component. J Cutan Pathol. 2012;39:781-6.
20. Closmann JJ, Eliot CA, Foss RD. Ectomesenchymal chondromyxoid tumor: Report of a case with description of histologic and immunohistochemical findings. J Oral Maxillofac Surg. 2013;71:545-9.
21. Seo SH, Shin DH, Kang HJ, Choi KU, Kim JY, Park DY, et al. Reticulated myxoid tumor of the tongue: 2 cases supporting an expanded clinical and immunophenotypic spectrum of ectomesenchymal chondromyxoid tumor of the tongue. Am J Dermatopathol. 2010;32:660-4.
22. van der Wal J, van der Waal I. Ectomesenchymal chondromyxoid tumor of the anterior tongue: Report of a case. J Oral Pathol Med. 1996;25:456-8.
23. Pires FR, Abrah�o AC, Cabral MG, Azevedo RS, Horta MCR, Martins CR, et al. Clinical, histological and immunohistochemical features of ectomesenchymal chondromyxoid tumor. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:914-9.
24. Ide F, Mishima K, Saito I. Ectomesenchymal chondromyxoid tumor of the anterior tongue with myxoglobulosislike change. Virchows Arch. 2003;442:302-3.
25. Concei��o JG, Gurgel CA, Ramos EAOG, De Aquino Xavier FC, Schlaepfer-Sales CB, Cangussu MCT, et al. Oral mucoceles: A clinical, histopathological and immunohistochemical study. Acta Histochem. 2014;116:40-7.
26. Tajima Y, Yokose S, Sakamoto E. Ameloblastoma arising in calcifying odontogenic cyst. Report of a case. Oral Surg Oral Med Oral Pathol. 1992;74:776-9.
27. Dickson BC, Antonescu CR, Argyris PP, Bilodeau EA, Bullock MJ, Freedman PD, et al. Ectomesenchymal Chondromyxoid Tumor A Neoplasm Characterized by Recurrent RREB1-MKL2 Fusions. Am J Surg Pathol. 2018;00:1-9.
28. Laco J, Mottl R, H�bling W, Ihrler S, Grossmann P, Skalova A, Ryska A. Cyclin D1 Expression in Ectomesenchymal Chondromyxoid Tumor of the Anterior Tongue. Int J Surg Pathol. 2016;24:586-94.
29. Schep LA, Bullock MJ, Taylor SM. Ectomesenchymal Chondromyxoid Tumour of the Dorsal Tongue Presenting with Impaired Speech. Case Rep Otolaryngol. 2016;2016:7342910.
30. AlZamel HA, AlBader A, Nawaz Bhat I. Ectomesenchymal chondromyxoid neoplasm. An unusual presentation. A case report. Int J Surg Case Rep. 2017:41:162-4.
31. Nigam S, Dhingra KK, Gulati A. Ectomesenchymal chondromyxoid tumor of the hard palate � A case report. J Oral Pathol Med. 2006;35:126-8.
32. Tajima S, Koda K. A case of a CD56-expressing ectomesenchymal chondromyxoid tumor of the tongue: Potential diagnostic usefulness of commonly available CD56 over CD57. Int J Clin Exp Pathol. 2015;8:3328-33.
�
Jean Nunes dos Santos
E-mail address: jeanunes@ufba.br
�
Ethical disclosures
Protection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of data. The authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consent. The authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
�
Conflict of interest
The authors have no conflicts of interest to declare.
�
Article history:
Received 28 January 2019
Accepted 21 May 2019
Available online 18 June 2019
