
Revista Portuguesa de Estomatologia, Medicina Dentária e Cirurgia Maxilofacial
SPEMD - Rev Port Estomatol Med Dent Cir Maxilofac | 2018 | 59 (4) | 221-224
Case report
Peters-plus syndrome: oral health approach
Síndrome de Peters-plus: abordagem odontológica
a Dental School, Federal University of Amazonas, Manaus, AM, Brazil
Emílio Carlos Sponchiado Júnior - spemilio@ufam.edu.br
Article Info
Rev Port Estomatol Med Dent Cir Maxilofac
Volume - 59
Issue - 4
Case report
Pages - 221-224
Go to Volume
Article History
Received on 03/09/2018
Accepted on 19/01/2019
Available Online on 06/02/2019
Keywords
Case report
�
Peters-plus syndrome: oral health approach
S�ndrome de Peters-plus: abordagem odontol�gica
�
Ma�ra Roberta Lima Viga, Em�lio Carlos Sponchiado J�nior*, Pollyanna Oliveira Medina, Ary de Oliveira Alves Filho, Simone Assayag Hanan
Dental School, Federal University of Amazonas, Manaus, AM, Brazil
�
�
http://doi.org/10.24873/j.rpemd.2018.11.422
�
Abstract
The aim of this paper is to report a clinical case of a male patient with Peters-plus syndrome. This syndrome is a rare, autosomal recessive congenital disorder. It is diagnosed by the presence of ocular changes associated with delayed psychomotor development, cardiac defects, and characteristic facial features, such as cleft lip, hypertelorism, narrow eyes, prominent forehead, and hearing loss. Clinical examination revealed gingival hyperplasia in the upper and lower arches resulting from the use of phenobarbital, associated with the presence of a large amount of dental biofilm, and active white spot lesions in the upper deciduous incisors. Replacement of the anticonvulsant medication was requested. Guidelines on oral and dietary hygiene habits as well as topical application of fluoride varnish were provided. Hyperplasia was reversed in 2 weeks and the patient has been receiving follow-up care. The patient is being monitored and his oral condition has improved.
Keywords: Cleft lip-palate,Dental care, Dentistry, Gingival hyperplasia,Peters anomaly
�
Resumo
O objetivo deste artigo � relatar um caso cl�nico de um paciente do sexo masculino com a s�ndrome de Peters-plus. Esta s�ndrome � uma doen�a cong�nita rara, autoss�mica recessiva, diagnosticada pela presen�a de altera��es oculares associadas ao atraso no desenvolvimento psicomotor, defeitos card�acos e caracter�sticas faciais pr�prias, como l�bio leporino, hipertelorismo, olhos estreitos, fronte proeminente e perda auditiva. O exame cl�nico revelou hiperplasia gengival nos arcos superior e inferior decorrentes do uso de fenobarbital, associada � presen�a de grande quantidade de biofilme dent�rio e les�es brancas ativas nos incisivos dec�duos superiores. Foi solicitada a substitui��o da medica��o anticonvulsivante. Diretrizes sobre h�bitos de higiene oral e alimentar, bem como aplica��o t�pica de verniz fluoretado foram implementadas. A hiperplasia foi revertida em 2 semanas e o paciente tem recebido acompanhamento. O paciente encontra-se em monitoriza��o e houve melhoria de sua condi��o oral.
Palavras-chave: Fenda palatina, Atendimento odontol�gico, Medicina dent�ria, Hiperplasia gengival,S�ndrome
de Peters
�
Introduction
Peters-plus syndrome, also known as Krause-Kivlin syndrome, is a rare congenital disorder of glycosylation with an autosomal recessive pattern. 1, 2 Its exact incidence is still unknown, and fewer than 75 cases with this anomaly have been identified in the medical literature.3
Peters-plus syndrome can be diagnosed clinically by the presence of Peters anomaly � a congenital corneal opacity secondary to a defect in neural crest cell migration that causes a malformation of the anterior eye segment, associated with other symptoms. These symptoms include a delayed psychomotor development, variable degrees of mental retardation, disproportionate short stature, and some facial features such as cleft lip or palate (present in about half of the cases), hypertelorism, narrow eyes, prominent forehead, a thin/cupid�s bow-shaped upper lip, long philtrum, small ears, hearing loss, broad/webbed neck, and joint hyperextensibility. The presence of congenital heart defects, genitourinary abnormalities such as cryptorchidism, hypoplastic clitoris and hydronephrosis, as well as structural brain malformations may affect the prognosis of individuals. Homozygous mutations of the B3GALTL gene in the 13q12.3 region have been associated with this phenotype.4 Toxic, infectious and traumatic events have also been suggested as possible causes of this syndrome.5
Little is known about this syndrome and studies in the scientific literature, particularly in the dental field, are scarce.
Thus, knowing and identifying its main clinical and oral manifestations is of great importance to establish an early diagnosis and rehabilitation, as well as referring families to genetic counseling. 2, 5 - 6
The dental treatment of these patients must especially take into account the degree of mental retardation, the clinical and oral manifestations of the disease, such as possible cardiac anomalies, and the oral impact of the medications administered.
The present study reports a case of a patient with Peters-plus syndrome submitted to dental treatment.
Case report
The 15-month-old male patient, B.S.M, diagnosed with Peters- plus syndrome, was brought to the School of Dentistry of the Federal University of Amazonas by his paternal grandmother, with the main complaint of �gingival swelling� since birth. During anamnesis, the legal guardian informed us that she had been referred by the neurologist and that the child had been born with hydrocephalus, facial changes, and cleft lip/palate.
The patient had already undergone 10 surgical interventions, including cheiloplasty to correct the cleft lip at the age of 6 months, two interventions for the implantation of ventriculoperitoneal shunt valves to relieve intracranial pressure, two to correct an inguinal hernia, two surgeries for eyelid reconstruction, and three external and middle ear surgeries.
The patient had undergone cardiac examinations for the diagnosis of possible heart abnormalities, which are commonly associated with the syndrome, but no abnormalities were found. There was no report of food or medication hypersensitivity.
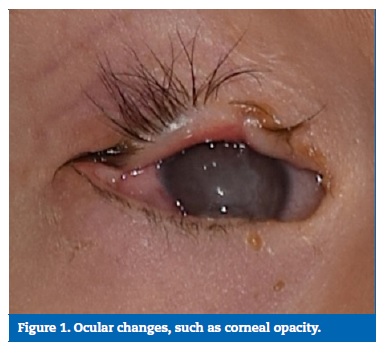
The anticonvulsant drug Gardenal� (40mg/mL) had been continuously administered orally twice a day over the previous 10 months, together with vitamin C. At the extraoral clinical examination, the syndrome�s typical facial appearance was observed, including bilateral corneal opacity (Figure 1), which was more visible in the right eye with consequent vision reduction, short stature, delayed psychomotor development and hearing loss.
�
�
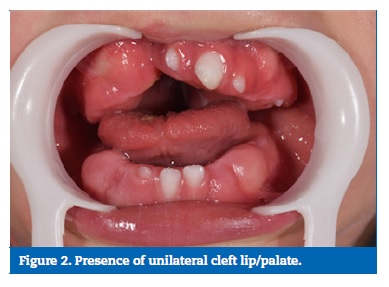
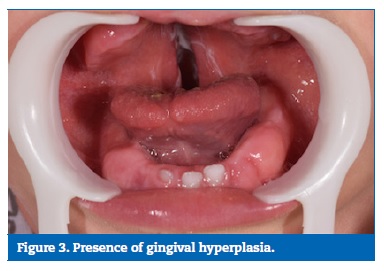
The intraoral examination revealed the presence of unilateral cleft lip and palate on the right side, gingival hyperplasia in the upper and lower arches, and gingival bleeding on brushing due to phenobarbital, associated with the presence of large amounts of dental biofilm, and active white spot lesions in the deciduous upper incisors (Figures 2 and 3).
�
�
�
Initially, a letter was sent to the neurologist of the patient requesting replacement of the medication. Oral hygiene instructions and dietary advice were provided, including solid foods� introduction and sugar�s rational use, considering that the infant was solely fed on pasty food and liquids.
The following week, phenobarbital was replaced by carbamazepine.7 Dental prophylaxis with Clinpro� prophylactic paste (3M, S�o Paulo, SP, Brazil) and 4 applications of fluoride varnish (Duraphat�, Colgate-Palmolive Ind. And Com. Ltda, S�o Paulo, SP, Brazil), one application per week, were planned with the purpose of enhancing the remineralization of the active white spot lesions.8-10 A 0.12% chlorhexidine digluconate mouthwash was prescribed to be used twice a day for 7 days after toothbrushing, administered with a sterile gauze swab, as the patient�s age contraindicated mouth washing. 11 - 12
Two weeks after drug replacement and hygiene and dietary guidance, an improvement in the presence of biofilm and aspect of the gingival tissue were observed. The patient has been followed up, and quarterly visits to the dental office have been scheduled with the purpose of improving the family�s and his quality of life.
Discussion and conclusions
Peters-plus syndrome is a rare congenital defect of glycosylation. According to the scientific literature, it is associated with mutations in the B3GALTL gene, located at chromosome 13.5 Therefore, the patient was under genetic follow-up to investigate the sequential analysis of the gene and the molecular diagnosis of a possible mutation; when the latter is not identified, deletion or duplication should be investigated. 3, 6
On the other hand, studies have reported cases of Peters-plus syndrome with characteristic clinical manifestations and lack of mutation of the B3GALTL gene. 4, 13 However, in these cases, not all the typical characteristics of the syndrome are observed. The importance of the molecular diagnosis of possible mutations is related to genetic counseling for future pregnancies.3 Uncovering its causes, which are still unclear, represents a potential chance for enhanced treatment and prevention through genetic counseling or possible preventive measures during pregnancy.5
The clinical diagnosis of this syndrome is usually based on the presence of ocular abnormalities, cleft lip and palate, facial changes, short stature and variable degrees of psychomotor delay, which corroborate the clinical findings of the present case. It may also be associated with conductive hearing loss, which was present in this patient as well, since he had congenital small malformed ears and had already undergone surgical interventions for the correction of external (pinna) and medium ears. Cardiac malformations may also occur in approximately 33% of cases3 but they were not observed in this infant, as no cardiac defects had been diagnosed.
Dental treatment varies according to the patient�s health condition, and early detection of the disease is important for early treatment to ensure good development of the general and oral health of the child.
In this case, dietary counseling and remineralization of the active white spot lesions were proposed. This relationship is consistent with the sugar-mediated pathobiology of dental caries. The high level of dental decay detected could be attributed to on-demand bottle feeding, high sweet consumption, poor oral hygiene, lack of use of fluoride prevention and lack of regular dental visits.14
Meta-analyses and systematic literature reviews document the efficacy of fluoride varnish application in inhibiting caries in primary teeth. 8, 10 In an effort to improve the consistency of preventive dental care, the American Academy of Pediatrics (AAP) recommends: (a) daily fluoride supplementation if the child�s drinking water is not fluoridated; and (b) fluoride varnish application after the emergence of the first tooth and every 6 months thereafter, receiving at least four treatments before the age of 4. 8, 15
Abnormal growth of gingival tissue resulting from adverse drug reactions has been observed in patients undergoing treatment with anticonvulsants, immunosuppressants and calcium channel blockers. However, knowledge about the pathogenesis of gingival growth is still limited,16 although it is acknowledged to be multifactorial, caused by disturbances in gingival fibroblasts.
Gingival hyperplasia or hypertrophy induced by anticonvulsants makes it difficult to maintain oral hygiene and, often, the masticatory function.17 To treat gingival hyperplasia, the medication may be replaced, after consulting the patient�s physician, and conservative periodontal treatment may be provided, including supra and subgingival scaling, prophylaxis and oral hygiene instructions. 7, 16
Although phenytoin is an anticonvulsant that causes gingival hyperplasia in about 40-50% of the patients who have been on the drug for at least 3 months, 18 - 19 primidone and, less frequently, valproate may also induce gingival growth in some patients.
Phenobarbital-induced gingival hyperplasia has been described by Lafzi, Farahani and Shofa as a rare condition.20 However, phenobarbital, similarly to phenytoin and primidone, is metabolized to 5- (4-hydroxyphenyl) 5-phenylhydantoin (4-HPPH). The serum concentration of this metabolite can explain the abnormal gingival growth caused by phenobarbital, which can be differentiated from that caused by other anticonvulsant drugs because it is uniform without lobulations of the interdental papillae.7
In the present case, we contacted the neurologist and phenobarbital was replaced by a new drug, which improved the clinical condition in 2 weeks.21 In cases of non-reversibility of the condition for 6 to 12 months, periodontal surgery may be performed to increase the clinical crown, such as gingivectomy/gingivoplasty, which is the most frequently used procedure.
It is often necessary to perform other surgeries to control recurrent growth. 7, 19 Patients who present with gingival hyperplasia should be carefully monitored, and meticulous oral hygiene is imperative.7 The patient is being monitored, and his oral condition has improved.
�
References
1. Hess D, Keusch JJ, Oberstein SA, Hennekam RC, Hofsteenge J. Peters Plus syndrome is a new congenital disorder of glycosylation and involves defective Omicron-glycosylation of thrombospondin type 1 repeats. J Biol Chem. 2008;283:7354-60.
2. Gupta N, Kaul A, Kabra M. Prenatal diagnosis of fetal Peters-plus syndrome: a case report. Case Rep Genet. 2013;2013:364529.
3. Bagul AS, Bokade CM, Saruk PV, Supare MS. Peters plus syndrome-like phenotype. J Clin Neonatol. 2015;4:193-5.
4. Siala O, Belguith N, Fakhfakh F. An unusual case of Peters plus syndrome with sexual ambiguity and absence of mutations in the B3GALTL gene. Iran J Pediatr. 2013;23:485-8.
5. Meyer I, Rolim H, Medeiros A, Paiva L, Galv�o Filho R. Anomalia de Peters, seus aspectos cl�nicos e terap�uticos: relato de caso. Arq Bras Oftalmol. 2010;73:367-9.
6. Schoner K, Kohlhase J, Müller AM, Schramm T, Plassmann M, Schmitz R, et al. Hydrocephalus, agenesis of the corpus callosum, and cleft lip/palate represent frequent associations in fetuses with Peters-plus syndrome and B3GALTL mutations. Fetal PPS phenotypes, expanded by Dandy Walker cyst and encephalocele. Prenat Diagn. 2013;33:75-80.
7. Hatahira H, Abe J, Hane Y, Matsui T, Sasaoka S, Motooka Y, et al. Drug-induced gingival hyperplasia: a retrospective study using spontaneous reporting system databases. J Pharm Health Care Sci. 2017;3:19.
8. Moyer VA, US Presentive Services Task Force. Prevention of dental caries in children from birth through age 5 years: U. S. Preventive services Task Force recommendation statement. Pediatrics. 2014;133:1102-11.
9. Steel K. How effective is the application of topical fluoride varnish in preventing dental caries in children? a literature review. Prim Dent J. 2014;3:74-6.
10. Twetman S, Dhar V. Evidence of effectiveness or current therapies to prevent and treat early childhood caries. Pediatr Dent. 2015;37:246-53.
11. American Academy of Pediatric Dentistry. Guideline on Management of Dental Patients with Special Health Care Needs. Reference Manual. 2016;38:171-6.
12. Dharmani CK. Management of children with special health care needs (SHCN) in the dental office. J Med Soc. 2018;32:1-6.
13. Weh E, Reis LM, Tyler RC, Bick D, Rhead WJ, Wallace S, et al. Novel B3GALTL mutations in classic Peters plus syndrome and lack of mutations in a large cohort of patients with similar phenotypes. Clin Genet. 2014;86:142-8.
14. Chi DL, Scott JM. Added Sugar and Dental Caries in Children: A Scientific Update and Future Steps. Dent Clin North Am. 2019;63:17-33.
15. American Academy of Pediatric Dentistry. Guideline on periodicity of examination, preventive dental services, anticipatory guidance/ counseling, and oral treatment for infants, children, and adolescents. Reference Manual. 2013:37:123-30.
16. Bharti V, Bansal C. Drug-induced gingival overgrowth: The nemesis of gingiva unravelled. J Indian Soc Periodontol. 2013;17:182-7.
17. Trackman PC, Kantarci A. Molecular and clinical aspects of drug-induced gingival overgrowth. J Dent Res. 2015;94:540-6.
18. Gurgel BC, de Morais CR, da Rocha-Neto PC, Dantas EM, Pinto LP, Costa Ade L. Phenytoin-induced gingival overgrowth management with periodontal treatment. Braz Dent J. 2015;26:39-43.
19. Devi PK, Kumar GP, Bai YD, Ammaji AD. Ipsilateral idiopathic gingival enlargement and it�s management using conventional gingivectomy and diode laser: a recurrent case after 15 years. J Indian Soc Periodontol. 2013;17:387-90.
20. Lafzi A, Farahani RM, Shoja MA. Phenobarbital-induced gingival hyperplasia. J Contemp Dent Pract. 2007;8(6):50-6.
21. Sharma PK, Misra AK, Chugh A, Chugh VK, Gonnade N, Singh S. Gingival hyperplasia: Should drug interaction be blamed for? Indian J Pharmacol. 2017;49:257-9.
�
Emilio Carlos Sponchiado J�nior
E-mail address: spemilio@ufam.edu.br
�
Ethical disclosures
Protection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of data. The authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consent. The authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
�
Conflicts of interest
The authors have no conflicts of interest to declare.
�
Article history:
Received 3 September 2018
Accepted 19 January 2019
Available online 6 February 2019
