
Revista Portuguesa de Estomatologia, Medicina Dentária e Cirurgia Maxilofacial
SPEMD - Revista Portuguesa de Estomatologia Medicina Dentária e Cirurgia Maxilofacial | 2025 | 66 (3) | 130-137
Original research
Antibiotic prescription habits of Portuguese oral healthcare professionals: an exploratory study
Hábitos de prescrição de antibióticos entre os profissionais de saúde oral portugueses: um estudo exploratório
a Universidade do Porto, Faculdade de Medicina Dentária, Porto, Portugal
b University of Porto, EPIUnit, Institute of Public Health, Porto, Portugal
c Universidade do Porto, Laboratory for Integrative and Translational Research in Population Health (ITR), Porto, Portugal
Álvaro Azevedo - aazevedo@fmd.up.pt
Article Info
Rev Port Estomatol Med Dent Cir Maxilofac
Volume - 66
Issue - 3
Original research
Pages - 130-137
Go to Volume
Article History
Received on 05/08/2024
Accepted on 23/07/2025
Available Online on 17/09/2025
Keywords
Original Research
�
Antibiotic prescription habits of Portuguese oral healthcare professionals: an exploratory study
H�bitos de prescri��o de antibi�ticos entre os profissionais de sa�de oral portugueses: um estudo explorat�rio
�
In�s Albuquerque1 0009-0004-6631-0156
Paulo Melo1,2,3 0000-0003-3590-4926
�lvaro Azevedo1,2,3* 0000-0002-1014-6148
1 Universidade do Porto, Faculdade de Medicina Dent�ria, Porto, Portugal.
2 University of Porto, EPIUnit, Institute of Public Health, Porto, Portugal.
3 Universidade do Porto, Laboratory for Integrative and Translational Research in Population Health (ITR), Porto, Portugal.
�
�
Article history:
Received 5 August 2024
Accepted 23 July 2025
Available online 16 September 2025
�
Abstract
Objectives: This research aims to characterize the antibiotic prescription patterns for adults with oral infections and their conditioning factors.
Methods: An online questionnaire was administered to dentists and stomatologists registered at the Portuguese Society of Stomatology and Dental Medicine (SPEMD) in 2023, and a non-random sample was obtained (n=85). The information obtained included sociodemographic and professional characteristics, as well as antibiotic prescription habits according to the Anatomical Therapeutic Chemical classification system. The binomial, chi-square, or Fisher�s exact tests were applied (α=0.05).
Results: The combination of amoxicillin and clavulanic acid (J01CR50) was the preferred choice of antibiotic of most participants (76.5%) and 95.3% (IC0.95 [89.2%-98.4%]) prescribed its recommended Defined Daily Dose. The recommended Defined Daily Doses were prescribed more frequently by professionals with less clinical experience (87.5%) (Fisher�s Exact test; p=0.018). Amoxicillin (J01CA04) was predominantly administered according to the recommended Defined Daily Dose by 71.8% of the participants (IC0.95 [61.6%-80.5%]), and azithromycin (J01FA10) by 76.5% of the participants (IC0.95 [66.7%-84.5%]). Fewer professionals working in the major centers (66.7%) prescribed the recommended Defined Daily Dose of azithromycin than in minor centers (87.5%) (χ2 = 5.108; df=1; p=0.024). Statistical significance differences were found between differently experienced professionals when faced with a diagnosis of irreversible pulpitis (χ2 = 4.587; df=1; p=0.032).
Conclusions: Portuguese oral health professionals generally prescribe antibiotics according to guidelines. However, a population-based study is essential to understand the detected disparities in priority antibiotic selection, Defined Daily Doses, and prescription habits in less obvious clinical situations. (Rev Port Estomatol Med Dent Cir Maxilofac. 2025;66(3):130-137)
Keywords: Antibiotics, Antimicrobial resistance, Dentists, Prescriptions,Stomatology.
�
Resumo
Objetivos: Caracterizar o padr�o de prescri��o de antibi�ticos e seus fatores condicionadores em adultos com infe��es orais.
M�todos: Foi aplicado um question�rio aos m�dicos dentistas e estomatologistas registados na Sociedade Portuguesa de Estomatologia e Medicina Dent�ria (SPEMD) em 2023, tendo-se obtido uma amostra n�o aleat�ria (n=85). Foram recolhidas caracter�sticas sociodemogr�ficas e profissionais, bem como h�bitos de prescri��o de antibi�ticos segundo a classifica��o farmacoterap�utica internacional
Anatomical Therapeutic Chemical. Foram aplicados testes do qui-quadrado ou exato de Fisher (α=0,05).
Resultados: A combina��o da amoxicilina com �cido clavul�nico (J01CR50) � escolha preferencial da maioria dos participantes (76,5%) e 95,3% prescreve de acordo com a quantidade di�ria de doses recomendadas (IC0.95 [89,2%-98,4%]). A quantidade di�ria de doses recomendadas � prescrita mais frequentemente por profissionais com menor experi�ncia cl�nica (87,5%) do que profissionais mais experientes (teste exato de Fisher; p=0,018). A amoxicilina (J01CA04) � prescrita de acordo com as doses di�rias recomendadas por 71,8% dos participantes (IC0.95 [61,6%-80,5%]), e a azitromicina (J01FA10) por 76,5% (IC0.95 [66,7%-84,5%]). Menos profissionais dos maiores centros (66,7%) prescrevem as doses di�rias recomendadas de azitromicina do que das restantes regi�es (87,5%) (χ2=5,108; df=1; p=0,024). Perante diagn�stico de pulpite irrevers�vel, foram detetadas diferen�as estatisticamente significativas entre profissionais com diferentes experi�ncias profissionais (χ2=4,587; df=1; p=0,032).
Conclus�es: Na generalidade, os profissionais de sa�de oral portugueses prescrevem antibi�ticos de acordo com as diretrizes. No entanto, � essencial um estudo de base populacional com maior representatividade, para compreender discrep�ncias na prioriza��o na sele��o dos antibi�ticos, doses di�rias recomendadas e h�bitos de prescri��o em situa��es cl�nicas controversas.
Palavras-chave: Antibi�ticos, Resist�ncia antimicrobiana, Dentistas, Prescri��es, Estomatologia.
�
Introduction
The discovery of penicillin by Alexander Fleming marked a groundbreaking moment in the history of medicine. Since then, the success of antibiotics has been unquestionable, reducing and improving morbidity and mortality worldwide.1, 2
Indeed, the success of penicillin paved the way for the development of numerous other antibiotics, expanding the range of treatable bacterial infections.
Antibiotic therapy has played a vital role in oral medicine for several decades.3 A study in Spain reported that 10% of all antibiotic prescriptions were meant for dental infections.4 This reflects the importance �of these medicines in managing oral infections and related dental procedures. Besides their primary role in treating oral cavity infections, these drugs also have additional benefits in dental practice: pain and discomfort management; prophylaxis for post-surgical complications (for a period of 5 to 7 days after surgery to prevent potential bacterial infections at the surgical site); combined with analgesics and anti-inflammatories; prophylactic approaches in high-risk patients, particularly for endocarditis.4 According to the recommendations of the American Heart Association, published in 2007 and updated in 2017, prevention of infective endocarditis is recommended only for those patients with the highest risk of adverse outcomes, combined with oral healthcare and regular access to dental care.5, 6
Prescription guidelines vary worldwide, depending on resistance patterns and accessibility to medical and dental services.7, 8 In Portugal, specific guidelines for antibiotic prescription in dental pathology have been established, notably through the Directorate General of Health (DGS) Standard 010/2014, published in 2014.9 Moreover, the Defined Daily Dose (DDD) metric has been used to monitor antibiotic consumption in the Portuguese population.10, 11
Despite the implemented guidelines, antibiotic use in dental practice is often associated with empirical prescriptions, 9 9 mostly for a very restricted range of broad-spectrum antibiotics for short periods.12, 13 This practice has been questioned, as empirical and possibly inappropriate prescriptions lead to the selection of resistant strains.14, 15 Therefore, dentists must exercise caution and adhere to responsible prescription practices.
Antibiotics should only be prescribed when necessary, and the choice of antibiotic, dosage, and duration of treatment should be based on sound clinical judgment and evidence-based guidelines.
The misuse and overuse of antimicrobials in animals are the main drivers in the growth of drug-resistant pathogens that contribute to developing antimicrobial resistance (AMR), a global public health concern.12 AMR is not just a prediction that could threaten our quality of life, but a reality that currently represents a significant concern worldwide.1 AMR is a leading global threat to public health and development. In 2019, it directly caused 1,27 million deaths worldwide and played a role in 4.95 million deaths.16 AMR occurs naturally over time and is an evolutionary process of microorganisms through successive genetic changes. However, the inappropriate use of antimicrobials accelerates this process,17 and that can only be overcome through correct antibiotic prescription. It is, therefore, importante to prevent the improper and/or unnecessary use of antimicrobials to increase the effectiveness of treatments. In this context, knowing the dentists� habits, perceptions, attitudes, and daily prescribing practices is paramount.18
While the awareness of clinical guidelines influences antibiotic prescription, non-clinical factors may also play a role.19
These factors include diagnostic uncertainty, explicit or implicit patient pressure, professional clinical experience, working conditions, sociodemographic characteristics of the population, and specific practices in different clinical specializations.20 - 22 Therefore, a nuanced, patient-specific approach, considering the severity of the infection and host factors, such as the immunological status, is of utmost importance to ensure responsible antibiotic use and mitigate the risk of AMR.
In 2021, the World Health Organization (WHO) declared AMR one of the top ten global public health threats facing humanity because the misuse and overuse of antimicrobial agents are the main drivers in developing resistant pathogens.12 Thus, antibiotics� efficacy is dissipating due to the ability of the bacterial microbiota to evolve and transform to resist them, minimizing their curative potential.1 In this context, medical professionals, including dentists and stomatologists, play a key role in the broader effort to address AMR by promoting prudent antibiotic use, staying informed about the latest guidelines and research in the field, and educating patients about its consumption.12
The current scarcity of information regarding antibiotic prescription among Portuguese oral health professionals demands more research. The results could play a pivotal role in raising awareness about the critical issue of AMR within the oral health community.
This pilot study aims to contribute to the characterization of antibiotic prescription habits for adult patients by oral health professionals registered in the Portuguese Society of Stomatology and Dental Medicine (SPEMD). It breaks down active ingredients, posologies, and prophylaxis according to geographical distribution, clinical experience, and clinical settings.
Material and methods
For this research, an online survey was conducted by email using the Google Forms� platform between February 14 and March 6, 2023. The target population was 2839 practicing dentists and stomatologists in Portugal registered in SPEMD. Nevertheless, only members with an updated email address in the list of registered professionals were invited to participate.
They received a detailed explanation of the study, including its aims and objectives, and had the principal investigator�s email address and telephone number available for any queries.
The questionnaire was developed based on previous studies with similar objectives identified through the bibliographic review.23 - 25 It was subsequently adapted to the Portuguese context, namely, the general clinical profiles of the professionals involved and the population density of the assessed regions.
Before its implementation, a pre-test was conducted on a sub-sample of ten volunteers to identify potential errors and clarify any doubts arising from imperfections in the questionnaire design.
The survey presentation indicated that an Ethics Committee had approved the research methodology. To proceed with the survey, participants were required to confirm that they had read the paragraph ensuring their confidentiality and anonymity and to declare their willingness to participate. A week before the online survey closed, an additional invitation to participate was sent as a reminder. Finally, a non-randomized sample of volunteer participants was collected.
Within the framework of this sociodemographic characterization study, comprehensive data acquisition encompassed variables such as sex, age (23-30; 31-40; 41-50; >50 years), clinical experience (<6; 6-10; 11-15; 16-20; >20 years), and the geographical region of clinical activity. To facilitate nuanced analysis, the different districts and the two autonomous regions were categorized into two distinct groups: major centers (Porto and Lisbon) and minor centers (the other 12 districts and autonomous regions).
To record the most frequently prescribed antibiotics, these were categorized according to the Anatomical Therapeutic Chemical (ATC) classification system:10 amoxicillin (J01CA04); amoxicillin with clavulanic acid (J01CR50); amoxicillin and metronidazole (J01R); cephalosporins (J01D); clarithromycin (J01FA09); clindamycin (J01FF01); tetracyclines (J01AA); azithromycin (J01FA10); metronidazole (J01XD01). For analyzing the posology pattern, the three main antibiotics for oral infections in adults were considered: amoxicillin, amoxicillin with clavulanic acid, and azithromycin. Therefore, parameters such as dosage, frequency of daily administrations, and overall treatment duration were registered as the usual amount of DDD administered: for amoxicillin 1000 mg tablets (0.5 DDD), the advised posology stood at 1000 mg, administered in two daily doses over eight days (8 DDDs); for amoxicillin with clavulanic acid 875 mg + 125 mg tablets (0.5 DDD), the advised posology was determined as 875 mg + 125 mg, administered twice daily for eight days (8 DDDs); for azithromycin 500 mg tablets (1 DDD), a dosage of once at 24-hour intervals over three days (3 DDDs) was considered.
The antibiotic prescription pattern was also evaluated in the most contentious clinical situations: endocarditis prophylaxis, before third-molar extraction, pulpitis diagnosis, nuclear diagnosis, and patient persuasion.
The statistical analysis included frequency analysis, confidence interval estimation for proportions, binomial test for a proportion of 0.5, and the chi-square test for independence. hen assumptions were not met, Fisher�s exact test was applied in tables (2X2) as an alternative. The level of significance was set at 5%.
The questionnaire was approved by the Ethics Committee of the Faculty of Dental Medicine of the University of Porto (ref. no. 27/2022, January 17, 2023) and by the Data Protection Unit of the University of Porto.
Results
A total of 1800 participation requests were successfully sent to the updated email address list of registered professionals, and a non-randomized sample of volunteer participants was obtained. Only responses from practicing oral healthcare professionals were deemed valid for the study (n=85), representing 5% of the inferential population. Within this cohort, 50.6% were male. The participants were 25 years or older, the most prominently represented age group was the >50 years (40%), and the least represented was the 23�30 years (8.2%). The most common clinical experience was over 20 years (49.4%), whereas the least was less than 6 years (7.1%). Regarding geographical distribution of clinical activity, 52.9% of participants practiced in the country�s two major centers (Lisboa and Porto), while the rest worked in smaller urban centers (Table 1).
Table 1. Sociodemographic and clinical characterization of the oral healthcare professionals
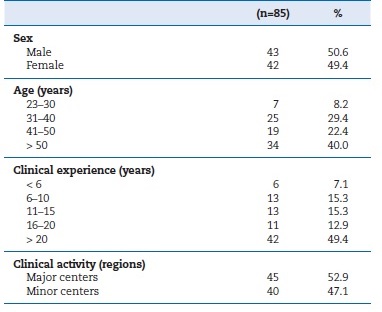
�
The majority of participants (76.5%) selected the combination of amoxicillin and clavulanic acid as their preferred choice of antibiotic, followed by plain amoxicillin (22.4%), and azithromycin (1.2%). Antibiotic prophylaxis involving the administration of 2 g of amoxicillin one hour before high-risk treatments was adopted by 81.2% of the participants (Table 2). Amoxicillin�s recommended DDDs were administered by 71.8% of the respondents, IC0.95 [61.6%-80.5%], and the observed difference was statistically significant (binomial test=0.5; p<0.001). In major centers, 80% of participants prescribed the recommended posology, while in minor centers, only 62.5% did; however, the difference was not statistically significant (χ2 = 3.201; df=1; p=0.074) (Table 3). Amoxicillin�s appropriate DDDs were prescribed by 62.5% of respondentes with ≤15 years of practice and by 77.4% of the remaining participants, with no statistically significant difference observed (χ2 = 2.174; df=1; p=0.14) (Table 3).
�
Table 2. Characterization of antibiotics used and endocarditis prophylaxis posology
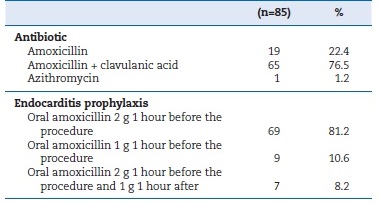
�
Table 3. Characterization of antibiotic prescription related to geographical region and clinical experience
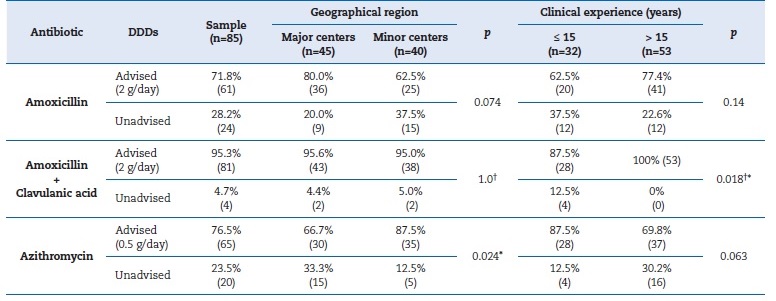
(p) P-value for chi-square test or alternatively Fisher�s exact test; (*) Statistically significant; (�) Fisher�s exact test; DDDS - Defined daily doses
Amoxicillin with clavulanic acid was administered according to the recommended DDDs by 95.3% of the participants, IC0.95 [89.2%-98.4%], and the observed difference was statistically significant (binomial test=0.5; p<0.001). The difference in the percentage of recommended DDDs between professionals from major centers (95.6%) and minor centers (95%) was 0.6%, respectively, not attaining statistical significance (Fisher�s exact test; p=1.0) (Table 3). On the other hand, 87.5% of professionals with less clinical experience prescribed this type of antibiotic appropriately, compared to the overall recommended prescriptions among more experienced professionals, achieving statistically significant differences (Fisher�s exact test; p=0.018) (TTable 3).
Azithromycin was administered according to the recommended DDDs by 76.5% of the respondents, IC0.95 [66.7%- 84.5%], and the observed difference was statistically significant (binomial test=0.5; p<0.001). Among professionals working in the major centers, 66.7% conventionally prescribed this antibiotic, while 87.5% of the remaining professionals did so, with statistically significant differences (χ2 = 5.108; df=1; p=0.024) (Table 3). Appropriate azithromycin DDDs were prescribed by 87.5% of professionals with less clinical experience, compared to the overall appropriate prescriptions among more experienced professionals, not attaining statistically significant differences (χ2 = 3.470; df=1; p=0.063) (Table 3).
When confronted with specific clinical conditions, such as before third-molar extraction, 81.3% of respondents with less clinical experience admitted to prescribing antibiotics, compared to 75.5% of the other professionals, although the difference found was not statistically significant (χ2 = 0.384; df=1; p=0.536) (Table 4). When faced with a diagnosis of pulpitis, 24.5% of respondents with more clinical experience admitted to prescribing antibiotics, compared to 6.3% in the group of less experienced professionals, achieving statistical significance (χ2 = 4.587; df=1; p=0.032) (Table 4).
When in doubt about the diagnosis, antibiotic prescription was more frequent among less experienced professionals than among more experienced ones, 71.9% and 64.2%, respectively, although the difference was not statistically significant (χ2 = 0.539; df=1; p=0.463) (Table 4). Among the participants, 34.1% admitted to prescribing antibiotics in response to patients� persuasion, with 31.3% among the less experienced professionals and 35.8% among the more experienced, without statistical significance (χ2 = 0.188; df=1; p=0.665) (Table 4).
�
Table 4. Characterization of the association between antibiotic prescription and years of clinical experience
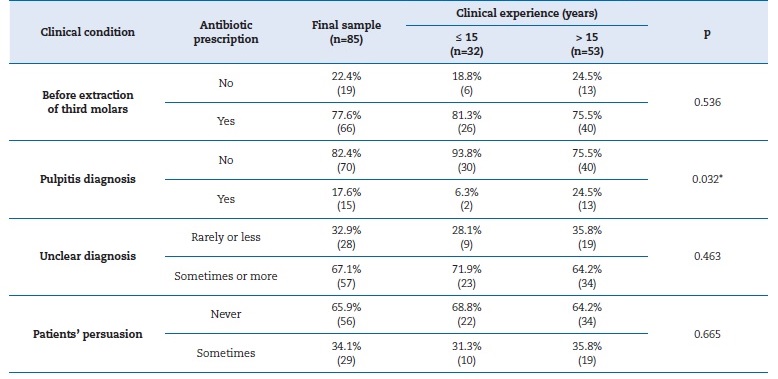
(p) P-value for chi-square test; (*) Statistically significant.
�
Discussion
Antibiotic resistance happens when bacteria evolve, making drugs less effective at treating infections. It has become one of the major public health threats of the 21st century. In this context, this study aims to analyze the antibiotic prescription habits of Portuguese oral health professionals treating adults.
The data collected show that the vast majority of participants do not prescribe the first-line antibiotics - amoxicillin alone - to treat oral bacterial infections. However, most prescribe the appropriate DDDs. On the other hand, for endocarditis prophylaxis, amoxicillin is prescribed properly: amoxicillin 2 g orally 1 hour before the clinical procedure.
Among the three main antibiotics prescribed, DDD prescription differences were found between the two groups of regions under study and among different years of clinical experience, although without statistical significance. Similarly, criteria for antibiotic prescription in the studied clinical situations also varied with clinical experience.
The prophylactic use of antibiotics is common to prevent post-surgical oral complications, and it mainly involves amoxicillin 1000 mg twice daily for eight days, associated with analgesics and anti-inflammatories,4 particularly in patients with concomitant pathologies, such as uncontrolled diabetes, or medically compromised patients.26 However, their use is most relevant in preventing bacterial endocarditis.3 For this purpose, the dose of amoxicillin 2 g one hour before dental treatment is the most appropriate27 to obtain a plasma concentration at therapeutic levels during the surgical procedure.5
In this study, 81.2% of the participating dentists revealed they chose the appropriate dosage, according to the American Heart Association (AHA) antibiotic prophylaxis guidelines.6 Similarly, a study in Spain on general dental practitioners found that the dosage of amoxicillin 2 g one hour before the appointment was the most common, although in a lower percentage than in the present study (71.1%).23
Traditionally, beta-lactam antibiotics (J01C) are the first choice to treat oral infections.28 In this study, the prescription of amoxicillin alone (22.4%) or combined with clavulanic acid (76.5%) was the first choice for patients not allergic to penicillin.
These findings align with reported prescription patterns of antibiotics in Portugal. According to Nunes and Matos,24 in a study covering 2015 to 2018, the most prescribed antibiotics within the penicillin group (J01C) in outpatient clinics were amoxicillin alone and amoxicillin with clavulanic acid. Over that period, there was a general uptick in the utilization of penicillin coupled with beta-lactamase inhibitors and macrolides (J01F).24 Other studies have also supported this trend, although with varying percentages between 45.6% and 61%.4, 29
However, it is important to note that this might not represente a universally agreed-upon pattern, as the prevalence of amoxicillin in monotherapy could be higher in other pathological contexts. In fact, many studies identified amoxicillin as the primary choice in several other countries, including Belgium (33.6%),14 Spain (ranging from 51.1% to 94.1%),23,25,28,30 United Kingdom (45.6%),31 United States of America (65.2%),32 Saudi Arabia (87.3%),33 and India (37%).34
By understanding and addressing the antibiotic prescription habits in the context of oral infectious diseases, this research seeks to contribute to the promotion of responsible antibiotic use, ultimately mitigating the risk of AMR in oral healthcare.18 According to the 2014 DGS recommendations,9 amoxicillin alone should normally be the first choice in dental pathology to avoid future AMR, 9 leaving the combination with clavulanic acid reserved for cases where beta-lactamases are suspected. Thus, that combination must be prescribed with caution, unlike what the results reflect, as it induces a greater risk of developing AMR.35
The efficacy of antibiotics depends on the corresponding DDD. Therefore, health professionals must carefully consider the dose administered and the time intervals so as not to compromisse the drug�s efficacy or exacerbate side effects, particularly by compromising the patient�s hepatic and renal homeostasis.9 Based on the dose regimens presented, and excluding the azithromycin 500 mg tablet due to its defined regimen of 3 DDDs, as well as more severe infections with variable treatment durations, it can be established that the duration of therapy with amoxicillin alone or with clavulanic
acid typically ranges from five to seven days. This duration should include an additional 48-hour period of antibiotic therapy after the symptom resolution, resulting in a total treatment duration of approximately eight days. 9, 13
Prescriptions according to the guidelines may vary depending on the accessibility to oral healthcare among diferente regions,36 as well as the clinical experience of oral healthcare professionals.22 In the penicillin group, including both amoxicillin alone and combined, appropriate dosages were more common among more experienced professionals, with statistical significance. Conversely, professionals practicing in smaller regions or who were less experienced were more likely to prescribe azithromycin in accordance with guidelines, although without statistical significance.
Surprising results were observed in the recommended posology between different regions: a higher frequency of macrolides (J01F) with appropriate DDDs, specifically azithromycin, was recorded in less densely populated regions (minor centers) with statistical significance. However, treatment periods exceeding three days of DDDs with azithromycin may be practiced in major centers. This finding may result from aiming to achieve greater efficacy in infection treatment due to the competitiveness experienced by professionals in large urban centers, as well as their perception of a higher frequency of bacterial resistance to antibiotics. This situation can be attributed to a greater turnover in the resident population due to tourism, migratory effects, and animal-derived foods.36
AMR is influenced not only by the antibiotics and corresponding DDDs consumed, but also by their actual necessity and specificity. An inappropriate treatment option identified in our study was the prescription of antibiotics for irreversible pulpitis. Without systemic manifestations, there is no indication for prescribing antibiotics in this condition.9 In these cases, endodontic treatment should be the first line of treatment.
Other studies also found prescription habits for pulpitis diagnoses, with lower percentages than in the present study, ranging between 4.3% and 14.1%.3, 13, 14, 31, 37 It should be emphasized that selecting appropriate treatment options for these conditions should be relatively easy.8
In this study, 67.1% of participants reported that they frequently prescribed antibiotics in situations of unclear diagnosis, underlining that there is still much insecurity about prescribing based on the guidelines, with concerns about AMR taking a back seat. In any case, Portuguese professionals faced with nuclear diagnosis have revealed inconsistent options regarding antibiotic prescription, which aligns with other studies in diverse countries. These variations are sometimes linked to a lack of familiarity with guidelines,19, 38 or the ambiguity of certain protocols, leading to the adoption of empirical practices in this field.39, 40 Conversely, professionals who prescribe beyond the guideline-recommended treatment often cite factors such as uncertainty in diagnosis and prognosis, limited time for surgical procedures, and patients� expectations regarding antibiotics.8
Regarding the latter argument and despite the need to respect patients� autonomy and opinions, oral health professionals should be prepared to refuse requests for a particular treatment if they believe it is not beneficial to the patient�s health, particularly when a patient requests an antibiotic without clinical justification.20 Accordingly, this research�s results are positive, given that most participants (65.9%) reported they had never been persuaded by requests for unnecessary antibiotic therapy.
Survey-based studies with volunteers tend to have low participation rates due to relying excessively on the cooperation of participants, especially regarding sensitive questions and knowledge testing. Those less convinced and usually less knowledgeable tend to refrain from participating. Research dependent upon voluntary subject participation is particularly vulnerable to sampling bias.41 Accordingly, the participation rate in this study was approximately 5%. Consequently, the sample size and sampling method do not permit us to ensure external validity. Nonetheless, the results obtained are consistent
with findings reported in other publications.3, 30, 34, 37 Thus, the current results should be indicative and a valuable starting point for larger nationwide studies. Besides raising awareness among the general population about the risk of consuming antibiotics autonomously, the results allow us to propose other measures:
1 � To carry out a broader population-based study broken down by professional sector.
2 � To understand the reasons for not applying the guidelines and sensitize professionals to apply them.
3 � To implement differentiated awareness-raising and training actions aimed at the rational use of antibioticsin this area.
Conclusions
In general, the oral health professionals who participated in this study prescribed antibiotics for adults in accordance with DGS and WHO guidelines. This adherence enhances their professional credibility and contributes to the prevention of AMR.
Nevertheless, a population-based study is crucial to better understand the observed discrepancies in priority antibiotic selection, recommended DDDs, and prescription practices in less straightforward clinical scenarios.
�
References
1. Sukumar S, Martin FE, Hughes TE, Adler CJ. Think before you prescribe: how dentistry contributes to antibiotic resistance. Aust Dent J. 2020;65:21-9.
2. Fluent MT, Jacobsen PL, Hicks LA, OSAP The Safest Dental Visit. Considerations for responsible antibiotic use in dentistry. J Am Dent Assoc. 2016;147:683-6.
3. D�Ambrosio F, Di Spirito F, Amato A, Caggiano M, Lo Giudice R, MArtina S. Attitudes towards Antibiotic Prescription and Antimicrobial Resistance Awareness among Italian Dentists: What Are the Milestones? Healthcare (Basel). 2022;10:1585.
4. Poveda-Roda R., Bagan J. V., Sanchis-Bielsa J. M., Carbonell-Pastor E. Antibiotic use in dental practice. A review. Medicina Oral, Patologia Oral y Cirugia Bucal. 2007;12:E186�E192.
5. Wilson W, Taubert KA, Gewitz M, Lockhart PB, Baddour LM, Levison M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2007;116:1736-54.
6. Wilson WR, Gewitz M, Lockhart PB, Bolger AF, DeSimone DC, Kazi DS, et al. Prevention of Viridans Group Streptococcal Infective Endocarditis: A Scientific Statement From the American Heart Association. Circulation. 2021;143:e963-78.
7. Thompson W, Teoh L, Hubbard CC, Marra F, Patrick DM, Mamun A, et al. Patterns of dental antibiotic prescribing in 2017: Australia, England, United States, and British Columbia (Canada). Infect Control Hosp Epidemiol. 2022;43:191-8.
8. Thompson W, Rios LE, Fedorowicz Z, Dailey Y, Douglas G. I�ve got Toothache, I need Antibiotics: a UK Perspective on Rational Antibiotic Prescribing by Dentists. Braz Dent J. 2018;29:395-9.
9. Dire��o Geral de Sa�de. Prescri��o de Antibi�ticos em Patologia Dent�ria. 2014. Available from: https://normas.dgs.min-saude.pt/2011/12/30/prescricao-de-antibioticos-empatologia-dentaria/. �Accessed 19 July, 2025.
10. WHO Anatomical Therapeutic Chemical (ATC) Classification. 2024. Available from: https://www.who.int/tools/atc-dddtoolkit/atc-classification. �Accessed 19 July, 2025.
11. Hutchinson JM, Patrick DM, Marra F, Ng H, Bowie WR, Heule L, et al. Measurement of antibiotic consumption: A practical guide to the use of the Anatomical Thgerapeutic Chemical classification and Definied Daily Dose system methodology in Canada. Can J Infect Dis. 2004;15:29-35.
12. WHO. Antimicrobial resistance. 2021. Available from: https://www.who.int/news-room/fact-sheets/detail/antimicrobialresistance. Accessed 19 July, 2025.
13. Tanwir F, Marrone G, Lundborg CS. Knowledge and reported practice of antibiotic prescription by dentists for common oral problems. J Coll Physicians Surg Pak. 2013;23:276-81.
14. Mainjot A, D�Hoore W, Vanheusden A, Van Nieuwenhuysen JP. Antibiotic prescribing in dental practice in Belgium. Int Endod J. 2009;42:1112-7.
15. Sweeney LC, Dave J, Chambers PA, Heritage J. Antibiotic resistance in general dental practice-a cause for concern? J Antimicrob Chemother. 2004;53:567-76.
16. Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399:629-55.
17. Emmott R, Barber SK, Thompson W. Antibiotics and toothache: a social media review. International J Pharm Pract. 2021;29:210-7.
18. Karasneh RA, Al-Azzam SI, Ababneh M, Al-Azzeh O, Al-Batayneh OB, Muflih SM, et al. Prescribers� Knowledge, Attitudes and Behaviors on Antibiotics, Antibiotic Use and Antibiotic Resistance in Jordan. Antibiotics (Basel). 2021;10:858.
19. Stein K, Farmer J, Singhal S, Marra F, Sutherland S, Qui�onez C. The use and misuse of antibiotics in dentistry: A scoping review. J Am Dent Assoc. 2018;149:869-84.e5.
20. Lewis PJ, Tully MP. The discomfort caused by patient pressure on the prescribing decisions of hospital prescribers. Res Social Adm Pharm. 2011;7:4-15.
21. Vazquez-Lago JM, Lopez-Vazquez P, L�pez-Dur�n A, Taracido-Trunk M, Figueiras A. Attitudes of primary care physicians to the prescribing of antibiotics and antimicrobial resistance: a qualitative study from Spain. Fam Pract. 2012;29:352-60.
22. Roberts RM, Bartoces M, Thompson SE, Hicks LA. Antibiotic prescribing by general dentists in the United States, 2013. J Am Dent Assoc. 2017;148:172-8.e1.
23. Dom�nguez-Dom�nguez L, L�pez-Marrufo-Medina A, Cabanillas-Balsera D, Jim�nez-S�nchez MC, Areal-Quecuty V, L�pez-L�pez J, et al. Antibiotics Prescription by Spanish General Practitioners in Primary Dental Care. Antibiotics (Basel). 2021;10:703.
24. Nunes AM, De Matos AA. Consumo de antibi�ticos em regime ambulat�rio: panorama portugu�s 2015-2018. Sa�de em Redes. 2022;8:329-45.
25. Segura-Egea JJ, Velasco-Ortega E, Torres-Lagares D, Velasco-Ponferrada MC, Monsalve-Guil L, Llamas-Carreras JM. Pattern of antibiotic prescription in the management of endodontic infections amongst Spanish oral surgeons. Int Endod J. 2010;43:342-50.
26. Munitic MS, Sutej I, Cacic N, Tadin A, Balic M, Bago I, et al. Knowledge and attitudes of Croatian Dentists Regarding Antibiotic Prescription in Endodontics: A Cross-sectional Questionnaire-based Study. Acta Stomatol Croat. 2021;55:346-58.
27. Nishimura RA, Carabello BA, Faxon DP, Freed MD, Lytle BW, O�Gara PT, et al. ACC/AHA 2008 guideline update on valvular heart disease: focused update on infective endocarditis: a report of the American College of Cardiology/American Heart Association Task Fosce on Practice Guidelines: endorsed by the Society of Cadiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons.. Circulation. 2008;118:887-96.
28. Rodriguez-N��ez A, Cisneros-Cabello R, Velasco-Ortega E, Llamas-Carreras JM, T�rres-Lagares D, Segura-Egea JJ. Antibiotic use by members of the Spanish Endodontic Society. J Endod. 2009;35:1198-203.
29. Kumar KP, Kaushik M, Kumar PU, Reddy MS, Prashar N. Antibiotic Prescribing Habits of Dental Surgeons in Hyderabad City, India, for Pulpal and Periapical Pathologies: A Survey. Adv Pharmacol Sci. 2013;2013:537385.
30. L�pez-Marrufo-Medina A, Dom�nguez-Dom�nguez L, Cabanillas-Balsera D, Areal-Quecuty V, Crespo-Gallardo I, Jim�nez-S�nchez MC, et al. Antibiotics prescription habits of Spanish endodontists: Impact of the ESE awareness campaign and position statement. J Clin Exp Dent. 2022;14:e48-54.136 rev port estomatol med dent cir maxilofac . 2025;66(3):130-137
31. Cope AL, Francis NA, Wood F, Chestnutt IG. Antibiotic prescribing in UK general dental practice: a cross-sectional study. Community Dent Oral Epidemiol. 2016;44:145-53.
32. Germack M, Sedgley CM, Sabbah W, Whitten B. Antibiotic Use in 2016 by Members of the American Association of Endodontists: Report of a National Survey. J Endod. 2017;43:1615-22.
33. Al-Johani K, Reddy SG, Al Mushayt AS, El-Housseiny A. Pattern of prescription of antibiotics among dental practitioners in Jeddah, KSA: A cross-sectional survey. Niger J Clin Pract. 2017;20:804-10.
34. Kaul R, Angrish P, Jain P, Saha S, Sengupta AV, Mukherjee S. A Survey on the Use of Antibiotics among the Dentists of Kolkata, West Bengal, India. Int J Clin Pediatr Dent. 2018;11:122-7.
35. Montagner F, Jacinto RC, Signoretti FGC, de Mattos VS, Grecca FS, Gomes BPFA. Beta-lactamic resistance profiles in Porphyromonas, Prevotella, and Parvimonas species isolated from acute endodontic infections. J Endod. 2014;40:339-44.
36. Frost I, Van Boeckel TP, Pires J, Craig J, Laxminarayan R. Global geographic trends in antimicrobial resistance: the role of international travel. J Travel Med. 2019;26:taz036.
37. Baudet A, Kichenbrand C, Pulcini C, Descroix V, Lesclous P, Thilly N, et al. Antibiotic use and resistance: a nationwide questionnaire survey among French dentists. Eur J Clin Microbiol Infect Dis. 2020;39:1295-303.
38. Angarita-D�az MDP, Bernal-Cepeda L, Rodriguez-Paz M, Vergara-Mercado M, Herrera-Herrera A, Forero-Escobar D, et al. Prescribing antibiotics by dentists in Colombia: Toward a conscientious prescription. J Public Health Dent. 2021;81:100-12.
39. Zahabiyoun S, Sahabi M, Kharazi MJ. Improving Knowledge of General Dental Practitioners on Antibiotic Prescribing by Raising Awareness of the Faculty of General Dental Practice (UK) Guidelines. J Dent (Tehran). 2015;12:171-6.
40. Marra F, George D, Chong M, Sutherland S, Patrick DM. Antibiotic prescribing by dentists has increased: Why? J Am Dent Assoc. 2016;147:320-7.
41. Cheung KL, Ten Klooster PM, Smit C, de Vries H, Pieterse ME. The impact of non-response bias due to sampling in public health studies: A comparison of voluntary versus mandatory recruitment in a Dutch national survey on adolescente health. BMC Public Health. 2017;17:276.
�
�lvaro Azevedo
E-mail address: aazevedo@fmd.up.pt
�
CRediT authorship contribution statement
In�s Albuquerque: Conceptualization, Data curation, Investigation, Methodology, Project administration, Visualization, Writing � original draft. Paulo Melo: Conceptualization, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization. �lvaro Azevedo: Conceptualization, Formal analysis, Methodology, Project administration, Software, Supervision, Validation, Visualization, Writing � original draft, Writing � review & editing.
�
Conflict of interest
The authors have no conflicts of interest to declare.
�
Ethical disclosures
Protection of human and animal subjects. The authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of data. The authors declare that they have followed their work center protocols on access to patient data and for its publication.
Right to privacy and informed consent. The authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
�
1646-2890/� 2025 Sociedade Portuguesa de Estomatologia e Medicina Dent�ria. Published by SPEMD.
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
