
Revista Portuguesa de Estomatologia, Medicina Dentária e Cirurgia Maxilofacial
SPEMD - Revista Portuguesa de Estomatologia Medicina Dentária e Cirurgia Maxilofacial | 2025 | 66 (3) | 124-129
Original research
An experience of oral cancer screening in the workplace: a cross-sectional study
Experiência de rastreio de cancro oral no local de trabalho: um estudo transversal
a Ahram Canadian University, Faculty of Oral and Dental Medicine, Department of Oral Medicine and Periodontology, Giza, Egypt
Ayat Gamal-AbdelNaser - ayat.gamal@dentistry.cu.edu.eg
Article Info
Rev Port Estomatol Med Dent Cir Maxilofac
Volume - 66
Issue - 3
Original research
Pages - 124-129
Go to Volume
Article History
Received on 09/08/2024
Accepted on 16/07/2025
Available Online on 11/09/2025
Keywords
Original Research
�
An experience of oral cancer screening in the workplace: a cross-sectional study
Experi�ncia de rastreio de cancro oral no localde trabalho: um estudo transversal
�
Ayat Gamal-AbdelNaser1,* 0000-0003-3564-8539
Mohamed Tariq Mohamed1 0009-0009-9873-8970
Aya Mohamed Ahmed1 0009-0000-8127-1715
Marina Ezzat Nabeh1 0009-0006-4083-3850
Mostafa Abdallah Anis1 0009-0000-2921-2814
Mahmoud Mohammed AbdelAziz1 0009-0004-5949-919X
1 Ahram Canadian University, Faculty of Oral and Dental Medicine, Department of Oral Medicine and Periodontology, Giza, Egypt.
�
�
�
Article history:
Received 9 August 2024
Accepted 16 July 2025
Available online 8 September 2025
�
Abstract
Objectives: Oral cancer is a serious disease whose prognosis worsens greatly with late detection and diagnosis. Therefore, a demand is pressing for a cost-effective model that is optimal for mass screening for oral cancer. So, this study aims to test oral cancer screening at the workplace to detect the prevalence of oral premalignant and malignant lesions in na institution-based campaign and to correlate it to the risk factors.
Methods: The cross-sectional study recruited all the employees of an institution. Information about the risk factors (smoking/ tobacco and alcohol use, sharp dental restorations) was gathered. Then, comprehensive intra-oral examination and lymph node examination were performed to detect any abnormalities.
Results: The study targeted 398 employees, 96 of whom declined participation. Therefore, oral screening was performed on 302 participants. The results showed zero oral malignancies, but oral findings were detected in 17 participants: frictional keratosis in 13 (4.3%), smoker�s keratosis in 2 (0.7%), and oral lichen planus in 2 (0.7%), which is an oral potentially malignant lesion. Chronic irritation and smoking were the only risk factors significantly associated with the detected lesions.
Conclusions: Oral cancer screening at the workplace was valuable in detecting the most common risk factors in the studied population. However, it revealed some embarrassment of the screened population that impaired the participation rate. Future studies should adopt some modification in the examination settings to overcome this limitation.
Keywords: Keratosis, Oral lichen planus, Precancerous conditions, Smoking.
�
Resumo
Objetivos: O cancro oral � uma doen�a grave cujo progn�stico se agrava muito com a dete��o e o diagn�stico tardios. Por conseguinte, � urgente encontrar um modelo rent�vel que seja ideal para o rastreio em massa de cancro oral. Assim, este estudo tem como objetivo testar o rastreio de cancro oral no local de trabalho para detetar a preval�ncia de les�es orais pr�-malignas e malignas numa campanha baseada numa institui��o e correlacionada com os fatores de risco.
M�todos: Este estudo transversal recrutou todos os funcion�rios de uma institui��o. Foram recolhidas informa��es sobre os fatores de risco. Em seguida, foi efetuado um exame intraoral completo e um exame dos g�nglios linf�ticos para detetar qualquer anomalia.
Resultados: O estudo visava 398 funcion�rios, 96 dos quais recusaram participar. Por conseguinte, foi efetuado um rastreio oral a 302 participantes. Os resultados revelaram zero tumores malignos orais, mas foram detetados achados orais em 17 participantes: queratose por fric��o em 13 (4,3%), queratose do fumador em 2 (0,7%) e l�quen plano oral em 2 (0,7%), que � uma les�o oral potencialmente maligna. A irrita��o cr�nica e o tabagismo foram os �nicos fatores de risco significativamente associados �s les�es.
Conclus�es: O rastreio de cancro oral no local de trabalho foi valioso na dete��o dos fatores de risco mais comuns na popula��o estudada. No entanto, revelou algum constrangimento da popula��o rastreada que prejudicou a taxa de participa��o. Estudos futuros devem adotar algumas modifica��es nos locais de exame para ultrapassar esta limita��o.
Palavras-chave: Queratose, L�quen plano oral, Condi��es pr�-cancer�genas, Tabagismo.
�
Introduction
Oral cancer represents the sixteenth most common malignant neoplasm worldwide. Among all types of oral cancer, 90% arise in the form of squamous cell carcinoma affecting the directly visible oral mucosa. The prognosis of the disease is expressed by its 5-year survival rate, which ranges from less than 50% to 80% depending on its stage at the time of detection.1
Even patients who are lucky to survive oral cancer face short-term and long-term complications due to the direct damage caused by the malignancy, post-operative surgical defects, and complications of radio- and chemotherapy, all together deteriorating their quality of life.2 Besides the fact that developing countries are twice as affected as developed ones, the economic burden of oral cancer was estimated as 75% of the gross domestic product per capita in some western countries.3 Adding to the above-mentioned physical and economic burden, unfortunately, the treatment modalities of oral cancer have not witnessed a substantial improvement in the past 30 years.
So, the main hope in improving the outcomes of the disease is focused on its prevention, early detection, and early treatment.4 The first approach for improving oral cancer outcomes is its prevention through eradicating its causes. Oral cancer derives from a complex combination of factors, involving several genetic and environmental risk factors.5 Common risk factos include tobacco (both smokeless and smoking) and alcohol consumption, areca nut chewing, older age, poor diet, infections, and persistent trauma.6
The second approach for better outcomes is early detection.
A significant reduction of 27% in oral cancer mortality and 24% in oral squamous cell carcinoma in high-risk groups was reported when they were early diagnosed.7, 8 Clinically, oral cancer is mostly preceded by oral potentially malignant lesions (OPMLs) like leukoplakia and erythroplakia. Generally, OPMLs affect high-risk patients, such as men over the age of 40, tobacco consumers, and alcohol abusers. However, individuals without classic risk factors may also present these lesions. Unlike what the name implies, only 5% of OPMLs transform into oral cancer. However, OPMLs at their early stages are difficult to detect by the patient as they do not cause symptoms.9 Therefore, oral screening plays a major role in promoting early diagnosis through the examination of apparently disease-free patients.8 It was proven to be valuable in identifying OPMLs and oral cancer, thus reducing the mortality and morbidity in highrisk patients and �down-staging� the disease.8
Screening has been employed through different models: general population-based screening, screening of high-risk populations only, opportunistic screening of dental patients at dental practices, oral screening integrated with general health checkups, screening at the workplace, and self-screening.1 Although population-based and risk-based screening models are the most commonly used in the literature, they suffer from low compliance levels of patients who attend the referral centers.
The model of screening at the workplace overcomes this limitation. However, this model was only previously applied and reported for workers in industrial sites.10
Therefore, this study aims to detect the prevalence of OPMLs and malignant lesions by adopting the model of screening at the workplace in an institution-based campaign and correlating the oral lesions to the risk factors. This testing represents preliminary efforts to find the best model for screening a general population for which oral cancer prevalence information is lacking.
Material and methods
This cross-sectional study was held during the first half of 2023 at a university campus with eight faculties. All employees working on the campus were interviewed. The study protocol is registered on Clinicaltrials.gov with the ID NCT06057857 and was approved by the institutional research ethics committee (Institutional
Review Board Organization IORG0010868) with the ID IRB00012891#31. The study followed the ethical standards of the
Declaration of Helsinki and its amendments. The research is reported following the guidelines of strengthening the reporting of observational studies in epidemiology (STROBE).11
The study team included senior dentistry students supervised by an Oral Medicine specialist. Before starting the screening campaign, all examiners performed repeated examinations and re-examinations of volunteers for validation and calibration. Inter-examiner and intra-examiner reliability was acceptable (Kappa coefficient >0.8).
All university employees were previously informed of the campaign and that their participation would be totally voluntary.
They were also alerted that the screening was to be performed at their offices, on their office chairs, to prevent any negative impact on their workflow.
The study team followed a systematic plan for the screening, where the team wandered through each room and passage of the university buildings in a certain order to ensure that they interviewed all employees. The study�s idea, aim, and procedures were explained to each employee. Only adults aged at least 18 years who granted informed consent were included in the study. Individuals who refused to participate were excluded. The employees were interviewed sequentially based on their office location to overcome any selection bias. After giving their informed consent, the participants were asked questions about having any of the risk factors (smoking/tobacco and alcohol use, sharp dental restorations) and history of systemic diseases, including cancer.
Comprehensive intraoral, pre-cervical lymph node, and cervical lymph node examinations were performed using gloves, a tongue depressor, and an artificial light source to detect any oral mucosal abnormality. The interviews were held through private one-to-one conversations, followed by the examination.
Intraoral examination included inspection and palpation of all oral mucosae, inspection of tonsils, and inspection and palpation of all teeth for any source of trauma. All steps were performed at the participant�s office.
The study tested for the following risk factors (predictors): (i) age; (ii) tobacco consumption (smoking and chewing) and number of cigarettes/day multiplied by number of years of smoking (cigarette.year); (iii) alcohol consumption; (iv) family history of oral cancer; and (v) chronic intraoral trauma. The outcomes included the presence of oral premalignant and malignant lesions, and correlating the risk factors with the detected lesions. Biopsy was planned in cases of suspected malignancy.
Each factor of the predictors and outcomes was statistically described according to its type. Continuous variables, like age and frequency of smoking, were expressed as mean and standard deviation, while categorical variables, like gender, educational level, and residence, were expressed as total number and percentage of the total. The distribution of the risk factors in relation to the detected lesions was tested for significance using the chisquare test with a significance level of p<0.05. The statistical analyses were performed using SPSS version 19 (IBM corp, NY).
The study is institution-based, where the whole population of employees was included, and no sampling was used for screening at the workplace.1 The total targeted population was 398.
Results
The study took place during February and March 2023 on the campus of a private university in Egypt. The study population encompassed 398 employees, of whom 96 declined participation due to embarrassment of being examined in front of colleagues and being busy working at the time of the screening campaign. Therefore, oral screening was performed on 302 employees (Figure 1).
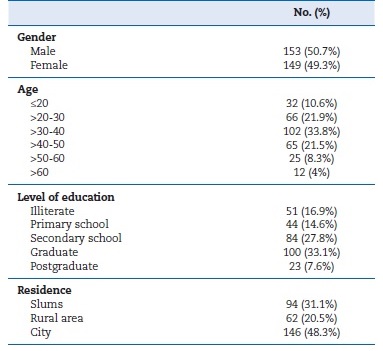
Regarding baseline data, the study included 153 males and 149 females, with a total mean age of 36.1 (�11.9) years. Most of the studied population was between 20 and 50 years of age and had between secondary school and university levels of education. This data is attributed to targeting employees who fit these criteria.
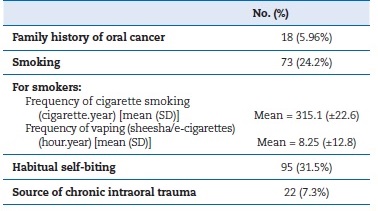
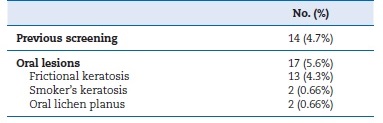
Table 1 shows the baseline demographic data of the participants. Regarding risk factors, the most prevalent risk factor was habitual biting (31.5%), followed by smoking (24.2%). Only 2 participants reported alcohol consumption (Table 2). Concerning the outcomes, only 4.7% of the screened sample reported previous screening for oral cancer (Table 3).
�
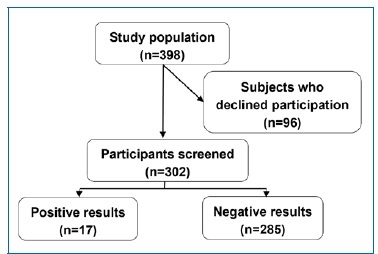
Figure 1. Flowchart showing the number of participants at each step of the study.
�
Table 1. Baseline data of the participants.
�
Table 2. Risk factor prevalence among the screened population.
�
Table 3. The prevalence of detected lesions.
�
No oral malignancies were detected, and only 0.66% of cases showed OPMLs in the form of oral lichen planus (OLP). None of the 5.6% who showed oral keratosis and OLP were aware of their lesion. Among the risk factors, both smoking and chronic intraoral trauma were significantly common among patients with oral lesions compared to those lesion-free (p=0.015 and p<0.01, respectively) (Table 4).
�
Table 4. Association of the risk factors with the detected lesions.

*P<0.05; **Tested by the chi-square test
�
Discussion
Oral cancer is a global health problem whose prevalence and mortality rates are increasing. Different oral lesions, known as OPMLs, have been reported to have the potential for malignant transformation.12 However, at early stages, oral cancer and OPMLs do not cause apparent symptoms and can pass unrecognized until late stages, when treatment options are more aggressive. Therefore, routine screening is recommended for early detection of unnoticed lesions.1, 13
Consequently, multiple models have been proposed and used for oral cancer screening. Unfortunately, each of the widely used models has limitations that render the results not completely satisfactory. Among the limitations is restricting the screening to individuals at risk, so campaigns are cost-effective, thus overlooking groups of individuals who are not categorized as high-risk groups. Therefore, a need is pressing for a screening model that is inclusive to all groups of individuals and, simultaneously, cost-effective.1
Although screening efforts have been reported in many countries as population- and community-based studies, rare reports are found from Egypt. Therefore, this study was the first study of screening at the workplace performed in Egypt.
The study targeted the employees of a private university who were screened at their offices.
Our results detected zero oral cancer cases, and 0.66% of the cases had OPMLs in the form of OLP. Besides the OPMLs, the screening detected frictional keratosis in 4.3% of the cases and smoker�s keratosis in 0.66%. Therefore, smoking and chronic trauma were the only risk factors that revealed a correlation with the occurrence of the detected oral lesions. Although frictional and smoker�s keratosis are not considered potentially malignant, smoking and chronic trauma are regarded as major risk factors for malignant transformation.
Furthermore, only 4.7% of the participants reported previous screening for oral cancer. To the best of our knowledge, there was only one study previously performed in Egypt for oral cancer and OPML screening.14 The hospital-based study only included smoker patients (cigarettes and shisha), including 1664 participants.
The study detected 27 cases of oral cancer (1.6%) and 107 case of OPMLs (6.4%): 59 cases of leukoplakia (3.5%) and 48 cases of OLP (2.8%). The higher number of cases detected compared to the present study can be attributed to the larger sample size and to exclusively including high-risk patients, namely, smokers.
Other than the Egyptian study, a Saudi study15 included only tobacco consumers (n=599), but only found a 3.9% prevalence of OPMLs with 2.3% leukoplakia, 0.7% erythroplakia, 0.5% oral submucous fibrosis, and 0.4% lichenoid reaction. On the other hand, 6.3% of the cases had oral keratosis. Similarly, a city-based Brazilian study8 that interviewed over 15,000 individuals and only included smokers, with a total participation of 202 individuals, found 70 lesions (34.7%) in total: 38 leukoplakia (18.8%), 17 actinic cheilitis (8.4%), 2 OLP (0.99%), and 1 OSCC (0.5%). The extremely high prevalence of OPMLs in this study is attributed to including only smokers.
Other studies had wider inclusion criteria with no restriction to smokers exclusively. A cross-sectional study in Georgia4 screened over 500 individuals attending a car racing event. The study detected suspicious lesions in 23% of the participants, but did not report the diagnoses of the lesions. However, they reported a prevalence of smoking (54%), alcohol consumption (43%), and smokeless tobacco use (13%) in the screened population.
Consequently, smoking was strongly correlated with the detected lesions. The high prevalence of tobacco and alcohol consumption explains the difference from the results of the current study. However, as the study did not report the diagnoses of the cases, it is impossible to compare its findings with any other study.
More detailed results were specified by a study targeting the Bangladeshi population in England.16 The study screened more than a thousand participants. The results revealed 9 cases of oral cancer (0.68%) and 8 cases of OPMLs (0.6%) in the form of 5 oral submucous fibrosis (0.38%) and 3 OLP (0.2%). The higher prevalence of malignant cases in this study may be attributed to the high prevalence of the reported risk factos like smoking cigarettes and bidi, together with chewing paan with and without tobacco. Such habits increase the risk of malignancies.
The common factor in all the above-mentioned studies is the screening model used, as all adopted the risk-based model. Each of the studies restricted the screening efforts to highrisk populations: either smokers,8, 14, 15 populations with high rates of tobacco and alcohol consumption,4 or disadvantaged populations. 16 Using the risk-based method was successful for detecting a considerable prevalence of OPMLs compared to no or a small percentage of oral cancer cases. Nonetheless, it skipped lesions in low-risk groups.
Conversely, the community-based model was used by a larger Indian study6 targeting a rural district and screening over 1 million participants. Despite the huge sample size, only 13 cases of oral cancer (0.001%) were detected, together with 174 cases of OPMLs (0.1%) in the form of leukoplakia (0.008%), submucous fibrosis (0.003%), and OLP (0.005%). In this study, tobacco smoking habit was identified in 62% of oral cancer patients, whereas 54% had the habit of chewing tobacco, and 69% were alcoholics. Another Indian study17 targeted the population of another district (4529 participants). They detected only one case of squamous cell carcinoma (0.02%) and 42 cases of OPMLs (0.9%), divided into 25 cases of leukoplakia and erythoplakia (0.55%), 12 cases of oral submucous fibrosis (0.3%), and 5 cases of tobacco pouch keratosis (0.1%). However, all lesions were detected exclusively in users of tobacco, either smoked or non-smoked.
Although the two Indian studies6 17 included massively larger sample sizes, the results showed comparable results to the current study regarding the percentage of potentially malignant lesions (0.1% and 0.9% versus 0.66% in our study). This similarity can be attributed to the fact that screening at the workplace targets the whole population of a certain institute, while the community-based model targets all inhabitants of a certain area. In other words, both models include a certain cluster as a whole without discriminating between the subjects based on their risk group. On the other hand, the huge sample sizes of the community-based studies implied a large burden, not only economically, but also in terms of the training of many healthcare workers to obtain the screening.6, 17, 18
Based on the results of all the aforementioned studies, screening of high-risk groups yields a higher number of oral cancer and OPML cases. Nonetheless, mass community-based screening succeeded in detecting cases that would be undetectable by other screening methods.
Although our study did not detect any malignancy and only detected a low prevalence of OPMLs, just like the community-based model, it shed light on the prevalence of risk factos of the screened population, namely smoking and chronic irritation.
The main highlight is that none of the detected cases acknowledged chronic irritation as a cause of a probable problem. Such cases would have been missed if screening had only been performed on high-risk groups.
The present study design met an unexpected limitation: the high rate of rejection to participate. This situation was attributed to the embarrassment of being examined in front of colleagues and being busy working during the screening campaign. Interestingly, low levels of participation were one of the main limitations also documented in community-based studies.19 However, in future studies adopting the model of screening at the workplace, such limitation can be overcome by avoiding screening at the participants� offices, and instead specifying a pre-planned and pre-announced schedule for participants in each department to be privately screened in a certain previously emptied office in their department.
Although the employers might not welcome that suggestion due to delaying the workflow of the employees, it would benefit the efficiency of the screening model while avoiding its limitations.
Other limitations cannot be overcome, as screening studies are inherently limited by the need to test risk factors and outcomes simultaneously. By doing so, the exact temporal sequence cannot be detected. So, the length of time needed for a lesion to develop cannot be concluded.20
Evidently, screening the whole population would not be cost-effective at large scales. However, this research recommends a nationwide series of institution-based screening programs, where all employees and workers would be screened as an integral part of their healthcare. Consequently, only positive cases would be referred to a specialist for investigation. Such fractionated efforts would end up adding to a large population-based screening program that includes the entire employed population. Embracing the nationwide fractionated plan would overcome the financial burden of large campaigns, skipping cases of screening high-risk groups exclusively, and the low participation levels. Associating that with opportunistic screening campaigns in dental hospitals would make a complete screening program na achievable goal.
Conclusions
Oral cancer screening in the workplace showed some unsuitability to the screened population. However, it was useful in detecting the most common risk factors in the working population of a certain institute, so that specific prevention programs can be planned targeting these risk factors. Further studies in multiple workplaces would sum up in a clearer image about the prevalence of OPMLs and oral cancer in the whole population.
�
References
1. Warnakulasuriya S, Kerr AR. Oral Cancer Screening : Past, Present, and Future. J Dent Res. 2021;100:1313-20.
2. Rezazadeh, F., Andisheh-Tadbir, A., Malek Mansouri, Z. et al. Evaluation of recurrence, mortality and treatment complications of oral squamous cell carcinoma in public health centers in Shiraz during 2010 to 2020. BMC Oral Health 23, 341 (2023).
3. Ribeiro-Rotta RF, Rosa EA, Milani V, Dias NR, Masterson D, da Silva EN, et al. The cost of oral cancer: A systematic review. PLoS One. 2022;17:e0266346.
4. Hapner ER, Wise JC. Results of a large-scale head and neck cancer screening of an at-risk population. J Voice. 2011;25:480-3.
5. Akinkugbe AA, Garcia DT, Brickhouse TH, Mosavel M. Lifestyle risk factor related disparities in oral cancer examination in the U. S : a population-based cross-sectional study. BMC Public Health. 2020;20:153.6. Philip PM, Nayak P, Philip S, Parambil NA, Duraisamy K, Balasubramanian S. Population ‑ based cancer screening through community participation : Outcome of a district wide oral cancer screening program from rural Kannur,. South Asian J Cancer. 2018;7:244-8.
7. Cheung LC, Ramadas K, Muwonge R, Katki HA, Thomas G, Graubard BI, et al. Risk-Based Selection of Individuals for Oral Cancer Screening. J Clin Oncol. 2021;39:663-74.
8. Pivovar A, dos Santos ZFDG, Torres-Pereira CC. Oral cancer screening for high-risk individuals in the primary healthcare setting using an active approach. J Oral Pathol Med. 2017;46:786-91.
9. Speight PM, Epstein J, Kujan O, Lingen MW, Nagao T, Ranganathan K, et al. Screening for oral cancer�a perspective from the Global Oral Cancer Forum. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;123:680-7.
10. Downer MC, Evans AW, Hallett CMH, Jullien JA, Speight PM, Zakrzewska JM. Evaluation of screening for oral cancer and precancer in a company headquarfers. Communiiy Dent Oral Epidemiol. 1995;23:84-8.
11. Von Elm E, Altman DG, Egger M, Pocock SJ, G�tzsche PC, Vandenbrouckef JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. Bull World Health Organ. 2007;85:867-72.
12. Gaphor DSM, Abdulla Sabri DZ. Prevalence of oral premalignant and malignant Lesions among referred Kurdish patients Attending Department of Oral and Maxilofasial in Sulaimani Teaching Hospital. IOSR J Dent Med Sci. 2014;13:32-6.
13. G�mez I, Warnakulasuriya S, Varela-Centelles PI, L�pez-Jornet P, Su�rez-Cunqueiro M, Diz-Dios P, et al. Is early diagnosis of oral cancer a feasible objective ? Who is to blame for diagnostic delay ? Oral Dis. 2010;16:333-42.
14. Abd el-Aziz A, AbouShousha A, ali shereen, Zahran F. Prevalence of Potentially Malignant Lesions and Oral Cancer Among Smokers in an Egyptian cohort: A Hospital-based Cross-Sectional Study. Adv Dent J. 2020;2:93-100.
15. Al-Attas SA, Ibrahim SS, Amer HA, Darwish ZES, Hassan MH. Prevalence of potentially malignant oral mucosal lesions among tobacco users in Jeddah, Saudi Arabia. Asian Pacific J Cancer Prev. 2014;15:757-62.
16. Nunn H, Lalli A, Fortune F, Croucher R. Oral cancer screening in the Bangladeshi community of Tower Hamlets : a social model. Br J Cancer. 2009;101(Suppl 2):S68-72.
17. Madankumar PD, Iyer K, Soni S, Nagarajan L, Kumar K, Solomon S, et al. A simple screening program for oral cancer in a defined geographic area in southern India: A community-based cross‑sectional study. Cancer Res Stat Treat. 2022;5:226-31.
18. Mandal R, Basu P. Cancer screening and early diagnosis in low and middle income countries: Current situation and future perspectives. Bundesgesundheitsblatt Gesundheitsforsch Gesundheitsschutz. 2018;61:1505-12.
19. Shrestha G, Gautam DK, Siwakoti B, Pradhananga KK, Mulmi R. Community-based Screening of Oral Cancer in Selected Districts of Nepal: A Cross-Sectional Study. Asian Pacific J Cancer Prev. 2023;24:4111-5.
20. Sartori LC, Fraz�o P. Accuracy of screening for potentially malignant disorders of the oral mucosa by dentists in primary care. Oral Health Prev Dent. 2012;10:53-8.
�
Ayat Gamal-AbdelNaser
E-mail address: ayat.gamal@dentistry.cu.edu.eg
�
Ethical disclosures
Protection of human and animal subjects. The authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of data. The authors declare that they have followed their work center protocols on access to patient data and for its publication.
Right to privacy and informed consent. The authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
�
Conflict of interest
The authors have no conflicts of interest to declare.
�
Acknowledgments
We would like to express great thanks and gratitude to graduates of the Ahram Canadian University Esraa Ahmed Ramadan Zahran, Haneen Osama Mohamed, Mohamed Reda Abd Elmaksoud, and Mohamed Nader Mahmoud, for their huge effort in this study.
�
CRediT authorship contribution statement
Ayat Gamal-AbdelNaser: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Supervision, Validation, Writing � review & editing. Mohamed Tariq Mohamed: Investigation, Methodology, Writing � original draft. Aya Mohamed Ahmed: Investigation, Methodology, Writing � original draft. Marina Ezzat Nabeh: Investigation, Methodology, Writing � original draft. Mostafa Abdallah Anis: Investigation, Methodology, Writing � original draft. Mahmoud Mohammed AbdelAziz: Investigation, Methodology, Writing � original draft.
�
1646-2890/� 2025 Sociedade Portuguesa de Estomatologia e Medicina Dent�ria. Published by SPEMD.
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
