
Revista Portuguesa de Estomatologia, Medicina Dentária e Cirurgia Maxilofacial
SPEMD - Revista Portuguesa de Estomatologia Medicina Dentária e Cirurgia Maxilofacial | 2025 | 66 (1) | 17-24
Original research
A 14-year retrospective study on clinical and histopathological features of epithelial salivary gland neoplasms
Estudo retrospectivo de 14 anos das características clínicas e histopatológicas de neoplasias epiteliais de glândula salivar
a School of Dentistry, Federal University of Rio Grande do Norte, Natal, RN, Brazil
b Department of Oral Pathology, Federal University of Rio Grande do Norte, Natal, RN, Brazil
c Department of Morphology, Biosciences Center, Federal University of Rio Grande do Norte, Natal, RN, Brazil
Lélia Batista de Souza - leliabsouza@gmail.com
Article Info
Rev Port Estomatol Med Dent Cir Maxilofac
Volume - 66
Issue - 1
Original research
Pages - 17-24
Go to Volume
Article History
Received on 17/05/2024
Accepted on 24/02/2025
Available Online on 27/03/2025
Keywords
Original Research
�
A 14-year retrospective study on clinical and histopathological features of epithelial salivar gland neoplasms
Estudo retrospectivo de 14 anos das caracter�sticas cl�nicas e histopatol�gicas de neoplasias epiteliais de gl�ndula salivar
�
Fl�via Luiza Santos Rodrigues1 0000-0002-8180-2675
D�bora Frota Colares2 0000-0002-8787-5904
Renata Roque Ribeiro2 0000-0002-1685-8955
Pedro Paulo de Andrade Santos3 0000-0002-5793-3886
L�lia Batista de Souza2,* 0000-0002-1277-3452
1School of Dentistry, Federal University of Rio Grande do Norte, Natal, RN, Brazil
2Department of Oral Pathology, Federal University of Rio Grande do Norte, Natal, RN, Brazil
3Department of Morphology, Biosciences Center, Federal University of Rio Grande do Norte, Natal, RN, Brazil
�
�
Article history:
Received 17 May 2024
Accepted 24 February 2025
Available online 27 March 2025
�
Abstract
Objectives: To investigate and describe the clinicopathological profile of pleomorphic adenoma, salivary adenoid cystic carcinoma, and mucoepidermoid carcinoma cases at a Northeast Brazilian Oral Pathology referral center.
Methods: Clinical features, histopathological diagnoses, subtypes, and specific histopathological features were collected from all cases previously diagnosed as pleomorphic adenoma, adenoid cystic carcinoma, or mucoepidermoid carcinoma between 2008 and 2021.
Results: Among 9613 cases diagnosed at the service, 86 (0.75%) were salivary gland neoplasms, included in this study. Of these, pleomorphic adenomas were the most common neoplasm (n = 49; 57.0%), followed by mucoepidermoid carcinomas (n = 23; 26.7%) and adenoid cystic carcinomas (n = 14; 16.3%). Patients ranged in age from 8 to 87 years old (mean age, 44.5 years), and the majority were female (65.3%). The palate was the most affected site in all salivary gland neoplasms. The predominant classifications were classical subtypes in pleomorphic adenomas (65.3%), cribriform and solid patterns in adenoid cystic carcinomas (42.8% both), and low-grade in mucoepidermoid carcinomas (n = 10; 43.5%). Adenoid cystic carcinoma cases were associated with pain (p < 0.001), age > 45 years (p = 0.024), and evolution time < 12 months (p = 0.019).
Conclusions: The findings of this study align with the literature. Although salivary gland neoplasms present overlapping clinical features, the association between clinical variables and adenoid cystic carcinoma diagnosis might aid clinical practice.
Keywords: Adenoid cystic carcinoma, Mucoepidermoid carcinoma, Pleomorphic adenoma, Salivary gland neoplasms
�
Resumo
Objetivos: Investigar e descrever o perfil clinicopatol�gico dos casos de adenoma pleom�rfico, carcinoma adenoide c�stico e carcinoma mucoepidermoide num centro de refer�ncia em Patologia Oral no Nordeste brasileiro.
M�todos: Caracter�sticas cl�nicas, diagn�sticos histopatol�gicos, subtipos e caracter�sticas histopatol�gicas espec�ficas foram recolhidos de todos os casos previamente diagnosticados como adenoma pleom�rfico, carcinoma adenoide c�stico ou carcinoma mucoepidermoide entre 2008 e 2021.
Resultados: Entre os 9613 casos diagnosticados no servi�o, 86 (0,75%) eram neoplasias de gl�ndulas salivares, inclu�dos neste estudo. Destes, os adenomas pleom�rficos foram as neoplasias mais comuns (n = 49; 57,0%), seguidos dos carcinomas mucoepidermoides (n = 23; 26,7%) e carcinomas adenoides c�sticos (n = 14; 16,3%). Os pacientes variaram de 8 a 87 anos de idade (idade m�dia, 44,5 anos), e a maioria era do sexo feminino (65,3%). O palato foi o local mais afetado em todas as neoplasias de gl�ndulas salivares. Houve predomin�ncia de subtipos cl�ssicos nos adenomas pleom�rficos (65,3%) e padr�es cribriforme e s�lido nos carcinomas adenoides c�sticos (ambos com 42,8%), enquanto a maioria dos carcinomas mucoepidermoides era de baixo grau (n = 10; 43,5%). Os casos de carcinoma adenoide c�stico estavam associados a dor (p < 0,001), idade > 45 anos (p = 0,024) e tempo de evolu��o < 12 meses (p = 0,019) em compara��o com outras neoplasias.
Conclus�es: Os achados deste estudo est�o alinhados com a literatura. Embora as neoplasias gl�ndulas salivares apresentem apar�ncia cl�nica sobreposta, a associa��o entre vari�veis cl�nicas e diagn�stico de carcinoma adenoide c�stico pode auxiliar na pr�tica cl�nica.
�
Palavras-chave: Carcinoma adenoide c�stico, Carcinoma mucoepidermoide, Adenoma pleom�rfico, Neoplasias de gl�ndulas salivares
�
Introduction
Salivary glands (SGs) are organs related to the gastrointestinal tract that produce and secrete saliva, a fluid with lubricating, digestive, immune, and homeostasis properties in the stomatognathic system.1 A wide range of genetic, inflammatory, reactive, and neoplastic diseases can occur in SGs.2, 3
The most recent edition of the World Health Organization (WHO) classification of head and neck tumors divides salivar gland neoplasms (SGN) according to their origin into benign and malignant neoplasms. The current classification includes more than 30 histological entities, of which some are the most frequently found in many populations.4 SGNs are rare, with na annual incidence of 0.5�2 patients/100,000 people,5 comprising about 2% of all head and neck tumors.6
Although SGNs are mostly found on major SGs, they may also appear in minor SGs and typically manifest as slow-growing asymptomatic swellings.7 Proper morphological analysis and diagnosis of SGN, following pre-established criteria, are necessary for accurate management and a favorable prognosis.
However, the diagnosis of these tumors is often challenging to pathologists due to the various and sometimes overlapping histopathological features among SGNs and their heterogeneity and diverse nature.8
Given the usual overlapping clinical and histopathological characteristics among SGNs, this research aims to study the histopathological and clinical features of three main SGNs: pleomorphic adenoma (PA), salivary adenoid cystic carcinoma (ACC), and mucoepidermoid carcinoma (MEC).
Materials and Methods
The Research Ethics Committee of the Federal University of Rio Grande do Norte approved the study (Approval No. 5.361.842). All cases of PAs, ACCs, and MECs diagnosed from January 2008 to December 2021 at an Oral Pathology referral center were retrieved and reviewed. Clinical data (patients� sex and age; lesions� anatomic location, clinical aspect, size, and color; and symptoms, duration, and clinical diagnosis) were collected from the corresponding biopsy records.
Sections were cut with a 5-μm thickness, deparaffinized, and stained with hematoxylin-eosin (H&E) for morphological examination under a light microscope (Five-Head Microscope, Nikon Eclipse-E200, Tokyo, Japan). PA samples were analyzed based on the system proposed by Seifert et al.9 (classic, stroma-rich, and cellular). ACCs� categorization (cribriform, tubular, and solid) and MECs� histopathological grading system followed the last WHO classification.2 Moreover, all SGNs included underwent a detailed morphological analysis (Table 1). In PAs, non-luminal cell morphology was analyzed based on criteria proposed by Dardick10 and Ellis and Auclair,11 and tumor capsule was described according to Lopes et al.12
�
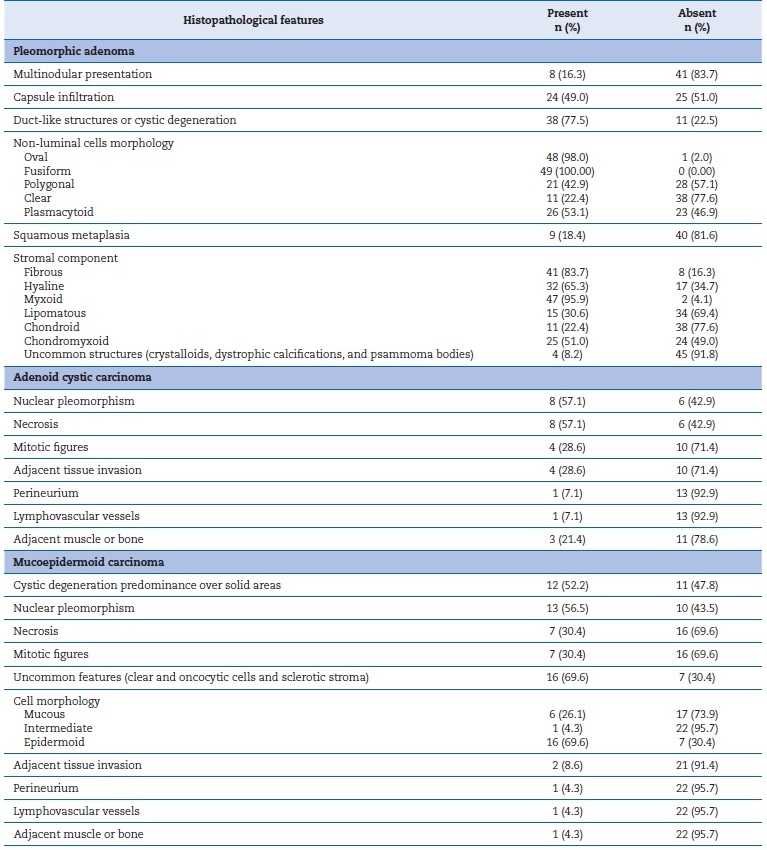
Table 1. Histopathological features observed in pleomorphic adenomas, mucoepidermoid carcinomas, and adenoid cystic carcinomas.
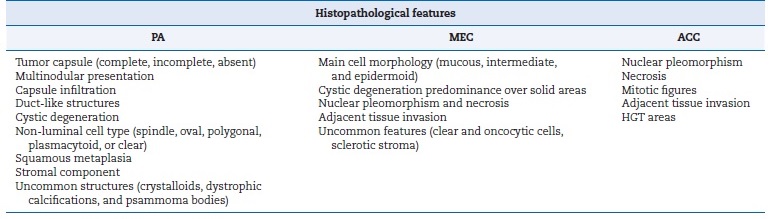
PA � pleomorphic adenoma; ACC � adenoid cystic carcinoma; MEC � mucoepidermoid carcinoma; HGT � high-grade transformation.
�
Descriptive statistics were computed for all study variables. Fisher�s exact test was performed to observe associations between tumors and their clinicopathological features.
All data analyses were performed using IBM SPSS Statistics version 22.0 (IBM, Chicago, IL). A p value ≤ 0.05 was considered statistically significant for all analyses.
�
Results
Among 9.613 cases diagnosed at the service between 2008 and 2021, 86 (0.75%) were SGNs and were included in this study. Of these, the most common neoplasm was PA (n = 49; 57.0%), followed by MEC (n = 23; 26.7%) and ACC (n = 14; 16.3%).
There was a female predominance among PAs and MECs; in ACCs, both sexes were equally affected. Most of the patients ranged from 30 to 59 years old. Regarding evolution time at diagnosis, considering only available data, more than 50% of PA cases had a duration longer than 12 months. Malignant lesions� evolution time ranged widely, mostly shorter than 12 months. Pain was present in 11.4% of PAs and more than 40% of all malignant lesions (Table 2).
�
Table 2. Absolute and relative distribution of demographic and clinical features of the salivary gland neoplasms included.
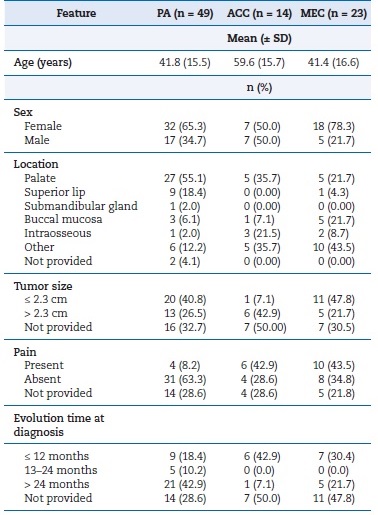
PA � pleomorphic adenoma; ACC � adenoid cystic carcinoma;
MEC � mucoepidermoid carcinoma.
�
Considering only available data, PAs� clinical presentation was mostly as normochromic (51.0%) nodules (38.8%) smaller than 2.3 cm (40.8%) affecting minor SGs, with a predilection for the palate, followed by the superior lip and the buccal mucosa.
The palate was also the most frequent site of ACCs, which frequently manifested as reddish (35.7%) ulcers (28.8%) �larger than 2.3 cm (42.9%). Regarding MECs, a wide range of sites were seen, with a predominance in the palate and the buccal mucosa. These carcinomas had a normochromic (34.8%) nodule (34.8%) appearance and usually were no larger than 2.3 cm (47.8%). Intraosseous cases comprised about 21% of ACCs, while almost absent in PAs and MECs (Table 2).
Patients younger than 45 years were mostly affected by PAs or MECs, while those older than 45 years were statistically associated with ACC diagnosis (p = 0.019). Moreover, pain was significantly more present in ACCs than in the other two groups (p < 0.001). Differences regarding evolution time were also observed, being longer for PAs and shorter for ACCs (p = 0.024) (Table 3).
�
Table 3. Clinical features and their differences according to the salivary gland neoplasms included.
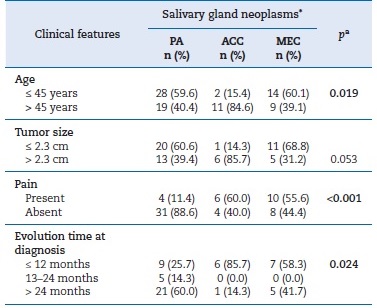
PA � pleomorphic adenoma; ACC � adenoid cystic carcinoma; MEC � mucoepidermoid carcinoma.
aFisher�s exact test. Significant p values are in bold (p < 0.05).
*Percentage was calculated based on available data for each variable.
�
Histopathological analysis of PAs revealed epithelial and myoepithelial cells forming islands, nests, chords, and ductlike structures distributed in varying types of stromal tissue.
PAs were often classified as a classical subtype (~65%), followed by cellular (~18.4%) (Table 4, Figures 1 A, B, and C). Table 5 shows the parenchymal and stromal features in detail. A common and well-known feature was the presence of a capsule, seen in almost 60% of cases, of which 40% were incomplete. About 70% of these lesions revealed duct-like structures or cystic degeneration; cells were mostly oval or fusiform (Figures 1 A and C � insets). Additionally, about 50% of PAs exhibited capsular infiltration. Stromal content ranged widely, being most commonly myxoid and fibrous. There were no cases of bone tissue as a stroma component. Uncommon structures (crystalloids, dystrophic calcifications, and psammoma bodies) were observed in less than 8% of cases and multinodular presentation in about 15%.
�
Table 4. Absolute and relative distribution of the included salivary gland neoplasms according to their histopathological subtype or grading.
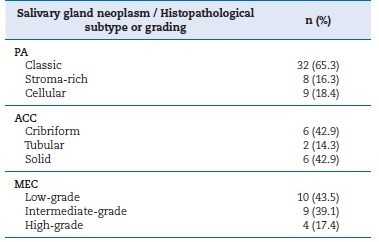
PA � pleomorphic adenoma; ACC � adenoid cystic carcinoma; MEC
� mucoepidermoidcarcinoma.
�
Table 5. Absolute and relative distribution of pleomorphic adenomas, adenoid cystic carcinomas, and mucoepidermoid carcinomas according to the histopathological findings observed.
�
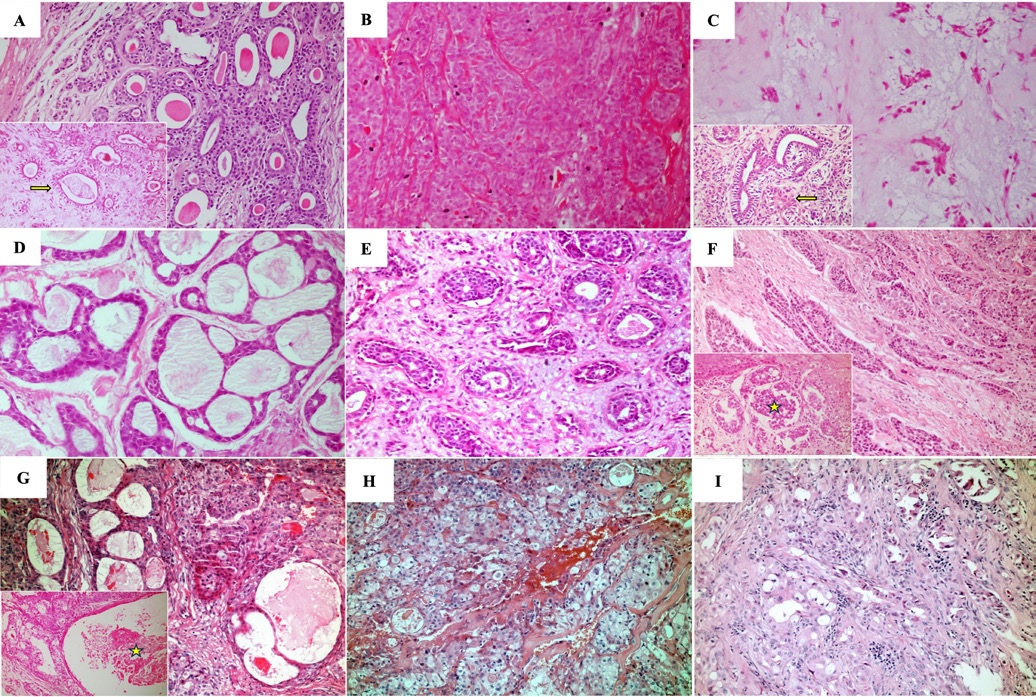
Figure 1. Histopathological features evidenced in the salivary gland neoplasms included. A) Classical pleomorphic adenoma exhibiting epithelial and myoepithelial cells forming duct-like structures (inset, yellow arrow) in a fibrous stroma (H&E, 100x and 200x). B) Cellular-subtype pleomorphic adenoma (H&E, 100x). C) Stroma-rich pleomorphic adenoma displaying epithelial and myoepithelial cells showing oval, fusiform, and polygonal morphology (inset, yellow arrow) in a myxoid stroma (H&E, 100x and 200x). D) Adenoid cystic carcinoma, cribriform subtype (H&E, 100x). E) Adenoid cystic carcinoma, tubular subtype (H&E, 100x). F) Solid subtype adenoid cystic carcinoma exhibiting necrosis (inset, yellow arrow) (H&E, 100x and 200x). G) Low-grade mucoepidermoid carcinoma exhibiting cystic degeneration areas (inset, yellow star) (H&E, 100x and 200x). H) Intermediate-grade mucoepidermoid carcinoma (H&E, 100x). I) High-grade mucoepidermoid carcinoma (H&E, 100x).
�
ACC cases were classified according to the most recente WHO classification.2 Cribriform and solid subtypes were the most common and exhibited similar prevalence (Table 4, Figures 1 D, E, and F). Table 5 describes the histopathological features of the ACCs analyzed in this study. Solid ACCs frequently exhibited cells forming solid patterns, nests, and islands, key features such as nuclear pleomorphism and mitotic figures, and areas of necrosis (Figure 1F � inset). Nuclear pleomorphism and necrosis were observed in at least 50% of cases.
Other common features of ACCs were also observed, and local tissue invasion was present in almost 29% of cases (n = 4). Only one tumor manifested as circumscribed. There were no ACCs with high-grade transformation in this study.
Finally, MECs were mostly categorized as low-grade, followed by intermediate (Table 4, Figures 1 G, H, and I). Table 5 details the histopathological analysis of MECs. Only 12.9% (n = 3) of the cases studied were well-circumscribed. Another important histopathological feature analyzed was cystic spaces in parenchyma, abundant in 50% of cases, at least when compared to solid cellular proliferation (Figure 1G � inset). Nuclear pleomorphism, necrosis, and mitotic figures were also common in MECs (Figure 1I). Only two cases (8.6%) had local tissue invasion.
Discussion
SGNs are known to exhibit clinical and histopathological overlapping features.8 Thus, this study investigated clinical and histopathological features of PAs, ACCs, and MECs. Overall, PAs were the predominant neoplasm, followed by MECs, which were the most common malignancy, as previously observed.6, 13, 14 ACCs were prevalent in patients over 45 years, while PAs and MECs were more common in younger individuals.
This finding is consistent with the literature, which states that ACCs are common in adults between the fifth and sixth decades of life, whereas MECs affect a broader age range and are the most common salivary malignancy in pediatric patients, with a peak incidence in the second decade of life.15, 16
Typically, major SGNs are diagnosed and treated in referral specialized medical centers because most SGNs occur in major SGs, while 9% occur in minor SGs, mostly in the palate.14, 17
This study was conducted with samples obtained from an Oral Pathology referral service, where a higher prevalence of SGNs in minor SGs is expected. Accordingly, all SGNs were mainly in the palate.
In this study, PAs manifested mostly as painless, normochromic nodules, which is consistent with previous studies.18, 19 This indolent behavior might be related to a longer evolution time at diagnosis, which was particularly associated with PAs compared to the other SGNs in this research. This lesion�s features may explain why patients might not seek treatment with the same urgency as when they have symptomatic diseases.
Microscopically, PAs were predominantly classical, aligning with Lopes et al.�s12 findings. Although this classification is apparently not clinically significant, it highlights the morphologic diversity of this neoplasm, which is important for its recognition and correct diagnosis. When located in major SGs, this neoplasm typically presents full and thick capsules.
In minor SGs, though, PAs commonly exhibit thin, incomplete, or malformed capsules.20, 21 Accordingly, in this study, the capsule was observed in almost 60% of cases, of which about 40% exhibited tumor infiltration in the capsule, as reported elsewhere.12, 18
PAs commonly exhibited fusiform and oval cells, as observed previously.18 Moreover, a prevalence of myxoid and fibrous stromal tissue was noted, aligning with the existing literature. Notably, the same PA might exhibit different types of stromal tissue.12, 18, 22 Despite a benign neoplasm with a predictable overall outcome, PAs might recur and undergo malignant transformation, named carcinoma ex-pleomorphic adenomas.
Histopathological analysis of PAs should consider features such as cell pleomorphism, necrosis, and atypical mitotic figures, although these alone do not signify malignancy.21, 23, 24In this study, no malignant features were observed in PAs; however, uncommon cell differentiation (clear and oncocytic cells) was present in approximately 20% of cases, as also observed by Lopes et al..12
Regarding ACCs, most cases manifest as a symptomatic ulcer. Notably, pain in malignant SGNs was also an importante feature, mainly observed in ACCs. The relationship between ACCs and pain and discomfort is well-acknowledged due to its invasive behavior, and commonly indicate advanced stages of the disease.15, 25 The presence of pain might also explain the shorter evolution time at diagnosis seen in this study compared to other SGNs.
Histopathological analysis of ACCs showed a high frequency of cribriform and solid subtypes. Although variations in the prevalence of histopathological subtypes are remarkably presente in the literature,26, 27 these findings align with previous reports.25 Some ACCs exhibited areas of islands and nests of tumor cells without any cell arrangements. While many studies have traditionally focused on the percentage of solid �reas for ACCs� classification and prognosis, Van Weert et al.28 highlighted that a solid pattern arranged in nests, islands, or sheets represents a prognostic factor. It is noteworthy that patients with solid ACCs are more susceptible to distant metastasis, advanced clinical stage, and lower 5-year and 10-year disease-free survival.25, 29, 30
Perineural invasion is another independent prognostic factor potentially linked to reduced overall and disease-free survival rates.31 Although a relatively common finding in ACCs, perineural invasion was observed in only 7% of cases in this study. The limited sample consisting solely of incisional biopsy specimens may account for the rarety of this finding. Noteworthy features observed in ACCs included cellular pleomorphism, mitotic figures, and areas exhibiting necrosis, as reported elsewhere.27 Severe nuclear atypia combined with factors such as desmoplastic stroma and expanded solid nests warrants consideration in histopathological analysis, as these indicate ACCs with high-grade transformation.32 In this study, no ACC had enough features to grade them as having highgrade transformation.
In this research, MECs manifested as normochromic nodules, consistent with the typical appearance of SGNs.33 SGNs� indistinguishable clinical appearance hinders their clinical diagnosis, except in cases where patients show pain and rapid growth, suggesting malignancy.34 Indeed, pain was one of the most remarkable features in MECs, observed in about 50% of cases. This finding is common in malignancies and is associated with invasive growth and compression of adjacent tissues and nerves near the neoplasm.35
Histologic grading of MECs revealed a prevalence of low and intermediate grades, consistent with previous studies.36, 37 Some factors associated with poor prognosis in these carcinomas include advanced TNM stage and histologic grading.38 Since the study samples were obtained from archives of an Oral Pathology referral service, only specimens from incisional biopsies were available, which prevented us from collecting additional information about other factors that could affect prognosis.
Diagnosing SGNs requires precision and expertise from qualified professionals. In minor SGs, diagnosing can be particularly challenging due to the high frequency of incisional biopsies, which may yield materials with insufficient information, and the prevalence of low-grade malignancies, complicating their differentiation from benign neoplasms.39 Ensuring that biopsies provide representative samples of lesions is essential to facilitate accurate diagnosis by pathologists.
In addition, many SG carcinomas exhibit histologic features that differ from well-recognized patterns, leading pathologists to diagnose them as �adenocarcinomas, no other specified (NOS).� New entities emerge with almost every updated WHO classification and are recognized based on morphologic and molecular patterns consistently reported in the literature.
Hence, observational studies aimed at describing morphological or molecular features of SGNs are necessary for recognizing and acknowledging new diseases, as well as for establishing better diagnostic criteria and management of these conditions.
The retrospective nature of this study implies certain limitations. Because the research was conducted at a single Oral Pathology referral service, the results may not fully reflect the overall clinicopathological profile of the included lesions. In addition, given the specific focus of this service, only selected intraoral SGNs (PAs, ACCs, and MECs) were analyzed in this study. Consequently, the findings of this study may not fully represent the biological and clinical behavior of the lesions studied, as they might also occur extraorally (e.g., parotid glands). Future research on the clinicopathological features of SGNs should include lesions on major and minor SGs, as well as other neoplasms within this group, to confirm or refine the findings of this study.
Conclusions
The overall findings of this study are aligned with the literature. Because the sample was retrieved from an Oral Pathology referral service, it is reasonable that most cases commonly occurred in the palate, which contrasted with previous reports. However, when only minor SGNs are considered, this finding corroborates previous reports.
In conclusion, SGNs exhibit a wide range of clinical and pathological features, which make their diagnosis a thorough and challenging process. Given the limitations of this study, further observational studies reporting epidemiological, clinical, and pathological data on SGNs, including other lesions within this group and occurrences in both major and minor SGs, are necessary to confirm or refine the present findings.
�
References
1. Paula F, Teshima THN, Hsieh R, Souza MM, Nico MMS, Louren�o SV. Overview of human salivary glands: highlights of morphology and developing processes. Anat Rec. 2017;300:1180-8.
2. WHO Classification of Tumours Editorial Board. Head and Neck Tumours. 5th ed. Volume 9. Lyon (France): International Agency for Research on Cancer; 2022.
3. Dos Santos ES, Rodrigues-Fernandes CI, Speight PM, Khurram SA, Alsanie I, Normando AGC, et al. Impact of tumor site on the prognosis of salivary gland neoplasms: a systematic review and meta-analysis. Crit Rev Oncol Hematol.2021;162:103352.
4. Sk�lov� A, Hyrcza MD, Leivo I. Update from the 5th edition of the World Health Organization classification of head and neck tumors: salivary glands. Head Neck Pathol. 2022;16:40-53.
5. To VSH, Chan JYW, Tsang RKY, Wei WI. Review of salivar gland neoplasms. ISRN Otolaryngol. 2012;2012:1-6.
6. Cunha JLS, Hernandez-Guerrero JC, De Almeida OP, Soares CD, Mosqueda-Taylor A. Salivary gland tumors: a retrospective study of 164 cases from a single private practice service in Mexico and literature review. Head Neck Pathol.2021;15:523-31.
7. Reinheimer A, Vieira D, Cordeiro M, Rivero E. Retrospective study of 124 cases of salivary gland tumors and literature review. J Clin Exp Dent. 2019;11:e1025-32.
8. Iyer J, Hariharan A, Cao UMN, Mai CTT, Wang A, Khayambashi P, et al. An overview on the histogenesis and morphogenesis of salivary gland neoplasms and evolving diagnostic approaches. Cancers (Basel). 2021;13:3910.
9. Seifert G, Langrock I, Donath K. A pathological classification of pleomorphic adenoma of the salivary glands (author�s transl). HNO. 1976;24:415-26.
10. Dardick I. Color atlas/text of salivary gland pathology. New York: Igaku-Shoin Medical Publishers; 1996.
11. Ellis GL, Auclair PL. Tumors of the salivary glands. Silver Spring: ARP Press; 2008.
12. Lopes MLDDS, Barroso KMA, Henriques �CG, Dos Santos JN, Martins MD, De Souza LB. Pleomorphic adenomas of the salivary glands: retrospective multicentric study of 130 cases with emphasis on histopathological features. Eur Arch Otorhinolaryngol. 2017;274:543-51.
13. Galdirs TM, Kappler M, Reich W, Eckert AW. Current aspect of salivary gland tumors � a systematic review of the literature. GMS Interdiscip Plast Reconstr Surg DGPW. 2019;8:Doc12.
14. Bruzinga FFB, Fernandes FCF, Dias FR, Lima MG, De Souza PEA, De Aguiar MCF, et al. Clinical and demographic features of minor salivary gland tumors: a collaborative study of 480 cases. Oral Dis. 2023;29:1028-38.
15. Nightingale J, Lum B, Ladwa R, Simpson F, Panizza B. Adenoid cystic carcinoma: a review of clinical features, treatment targets and advances in improving the immune response to monoclonal antibody therapy. Biochim Biophys Acta Ver Cancer. 2021;1875:188523.
16. Guevara-Canales JO, Morales-Vadillo R, Guzm�n-Arias G, Cava-Vergi� CE, Guerra-Miller H, Montes-Gil JE. Mucoepidermoid carcinoma of the salivary glands: a retrospective study of 51 cases and review of the literature. Acta Odontol Latinoam. 2016;29:230-8.
17. McKenzie J, Lockyer J, Singh T, Nguyen E. Salivary gland tumours: an epidemiological review of non-neoplastic and neoplastic pathology. Br J Oral Maxillofac Surg. 2023;61:12-8.
18. P�rez-de-Oliveira ME, Leonel ACLDS, De Castro JFL, Carvalho EJDA, Vargas PA, Perez DEDC. Histopathological findings of intraoral pleomorphic adenomas: a retrospective study of a case series. Int J Surg Pathol. 2019;27:729-35.
19. Almeslet AS. Pleomorphic adenoma: a systematic review. Int J Clin Pediatr Dent. 2020;13:284-7.
20. Nonitha S, Yogesh T, Nandaprasad S, Maheshwari Bu, Mahalakshmi I, Veerabasavaiah B. Histomorphological comparison of pleomorphic adenoma in major and minor salivary glands of oral cavity: a comparative study. J Oral Maxillofac Pathol. 2019;23:356.
21. Hernandez-Prera JC, Sk�lov� A, Franchi A, Rinaldo A, Vander Poorten V, Zb�ren P, et al. Pleomorphic adenoma: the great mimicker of malignancy. Histopathology. 2021;79:279-90.
22. Triantafyllou A, Thompson LDR, Devaney KO, Bell D, Hunt JL, Rinaldo A, et al. Functional histology of salivary gland pleomorphic adenoma: an appraisal. Head Neck Pathol. 2015;9:387-404.
23. Seok J, Hyun SJ, Jeong W-J, Ahn S-H, Kim H, Jung YH. The difference in the clinical features between carcinoma ex pleomorphic adenoma and pleomorphic adenoma. Ear Nose Throat J. 2019;98:504-9.
24. Piwowarczyk K, Bartkowiak E, Kosikowski P, Chou JT-T, Wierzbicka M. Salivary gland pleomorphic adenomas presenting with extremely varied clinical courses: a single institution case-control study. Front Oncol. 2021;10:600707.
25. Morais EF, Da Silva LP, Moreira DGL, Mafra RP, Rolim LSA, Santos EM, et al. Prognostic factors and survival in adenoid cystic carcinoma of the head and neck: a retrospective clinical and histopathological analysis of patients seen at a cancer center. Head Neck Pathol. 2021;15:416-24.
26. Bhayani MK, Yener M, El-Naggar A, Garden A, Hanna EY, Weber RS, et al. Prognosis and risk factors for early-stage adenoid cystic carcinoma of the major salivary glands. Cancer. 2012;118:2872-8.
27. Ben Salha I, Bhide S, Mourtzoukou D, Fisher C, Thway K. Solid variant of adenoid cystic carcinoma: difficulties in diagnostic recognition. Int J Surg Pathol. 2016;24:419-24.
28. Van Weert S, Bloemena E, Van Der Waal I, De Bree R, Rietveld DHF, Kuik JD, et al. Adenoid cystic carcinoma of the head and neck: a single-center analysis of 105 consecutive cases over a 30-year period. Oral Oncol. 2013;49:824-9.
29. Ishida E, Ogawa T, Rokugo M, Ishikawa T, Wakamori S, Ohkoshi A, et al. Management of adenoid cystic carcinoma of the head and neck: a single-institute study with over 25-year follow-up. Head Face Med. 2020;16:14.
30. Ikawa H, Koto M, Takagi R, Ebner DK, Hasegawa A, Naganawa K, et al. Prognostic factors of adenoid cystic carcinoma of the head and neck in carbon-ion radiotherapy: the impact of histological subtypes. Radiother Oncol.2017;123:387-93.
31. Ju J, Li Y, Chai J, Ma C, Ni Q, Shen Z, et al. The role of perineural invasion on head and neck adenoid cystic carcinoma prognosis: a systematic review and metaanalysis. Oral Surg Oral Med Oral Pathol Oral Radiol.2016;122:691-701.
32. Zhu S, Schuerch C, Hunt J. Review and updates of immunohistochemistry in selected salivary gland and head and neck tumors. Arch Pathol Lab Med. 2015;139:55-66.
33. Ogle OE. Salivary gland diseases. Dent Clin North Am. 2020;64:87-104.
34. Gatta G, Guzzo M, Locati LD, McGurk M, Prott FJ. Major and minor salivary gland tumours. Crit Rev Oncol Hematol. 2020;152:102959.
35. Witt RL. Major salivary gland cancer. Surg Oncol Clin N Am. 2004;13:113-27.
36. Byrd SA, Spector ME, Carey TE, Bradford CR, McHugh JB. Predictors of recurrence and survival for head and neck mucoepidermoid carcinoma. Otolaryngol Head Neck Surg. 2013;149:402-8.
37. Jeergal P, Karim Namazi N, Patil S, Kochar A, Sohoni R, Bussari S. Mucoepidermoid carcinoma: a retrospective clinicopathologic study of 25 cases. J Oral Maxillofac Pathol. 2021;25:490.
38. Fehr A, Werenicz S, Trocchi P, Falk M, Friedrich RE, Stammler A, et al. Mucoepidermoid carcinoma of the salivary glands revisited with special reference to histologic grading and CRTC1/3-MAML2 genotyping. Virchows Arch.2021;479:975-85.
39. Ihrler S, Agaimy A, Guntinas-Lichius O, Haas CJ, Mollenhauer M, Sandison A, et al. Why is the histomorphological diagnosis of tumours of minor salivary glands much more difficult? Histopathology.2021;79:779-90.
�
E-mail address: leliabsouza@gmail.com
�
CRediT authorship contribution statement
Fl�via Luiza Santos Rodrigues: Data curation, Formal analysis, Methodology, Investigation, Writing � original draft. D�bora Frota Colares: Data curation, Methodology, Investigation, Visualization, Writing � review & editing. Renata Roque Ribeiro: Visualization, Writing � review & editing. Pedro Paulo de Andrade Santos: Supervision, Writing � review & editing. L�lia Batista de Souza: Conceptualization, Funding acquisition, Investigation, Project administration, Resources, Supervision, Validation, Visualization, Writing � review & editing.
�
Conflict of interest
The authors have no conflicts of interest to declare.
�
Ethical disclosures
Protection of human and animal subjects. The authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of data. The authors declare that they have followed their work center protocols on access to patient data and for its publication.
Right to privacy and informed consent. The authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
�
Acknowledgements
This study was supported by the Postgraduate Program in Dentistry Sciences of UFRN and by the Coordination for the Improvement of Higher Education Personnel (CAPES). L.B.S. is a Research Productivity Fellow of the National Council for Scientific and Technological Development (CNPq).
�
1646-2890/� 2025 Sociedade Portuguesa de Estomatologia e Medicina Dent�ria. Published by SPEMD.
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
