
Revista Portuguesa de Estomatologia, Medicina Dentária e Cirurgia Maxilofacial
SPEMD - Revista Portuguesa de Estomatologia Medicina Dentária e Cirurgia Maxilofacial | 2024 | 65 (4) | 188-196
Original research
Effectiveness of three biomimetic remineralization procedures in artificial caries-affected dentin – an in-vitro pilot study
Eficácia de três procedimentos de remineralização biomimética na dentina afetada por cárie – estudo piloto in vitro
a Departamento de Dentisteria, Faculdade de Medicina Dentária, Universidade do Porto, Porto, Portugal
b Unidade de Microscopia Eletrónica, Universidade de Trás-os-Montes e Alto Douro, Vila Real, Portugal
c Departamento de Física e Astronomia, Faculdade de Ciências, Universidade do Porto, Porto, Portugal
d Departamento de Dentisteria, Faculdade de Medicina Dentária, Universidade do Porto, Porto, Portugal
e EpiUnit, ITR, Instituto de Saúde Pública, Faculdade de Medicina Dentária, Universidade do Porto, Porto, Portugal
Rosário Costa - mrcosta@fmd.up.pt
Article Info
Rev Port Estomatol Med Dent Cir Maxilofac
Volume - 65
Issue - 4
Original research
Pages - 188-196
Go to Volume
Article History
Received on 27/05/2024
Accepted on 07/12/2024
Available Online on 20/01/2025
Keywords
Original Research
�
Effectiveness of three biomimetic remineralization procedures in artificial caries-affected dentin � an in-vitro pilot study
Efic�cia de tr�s procedimentos de remineraliza��o biomim�tica na dentina afetada por c�rie � estudo piloto in vitro
�
Ros�rio Costa1,* 0000-0003-1022-6858
Sofia Arantes-Oliveira2 0000-0001-9105-0975
Lisete Fernandes3 0000-0002-2986-7818
F�bio G. Figueiras4 0000-0001-7841-9731
Jo�o Cardoso Ferreira1 0000-0003-4575-4861
Paulo Ribeiro de Melo1,5 0000-0003-3590-4926
1 Departamento de Dentisteria, Faculdade de Medicina Dent�ria, Universidade do Porto, Porto, Portugal
2 Departamento de Biomateriais, Faculdade de Medicina Dent�ria, Universidade de Lisboa, Lisbon, Portugal
3 Unidade de Microscopia Eletr�nica, Universidade de Tr�s-os-Montes e Alto Douro, Vila Real, Portugal
4 Departamento de F�sica e Astronomia, Faculdade de Ci�ncias, Universidade do Porto, Porto, Portugal
5 EpiUnit, ITR, Instituto de Sa�de P�blica, Faculdade de Medicina Dent�ria, Universidade do Porto, Porto, Portugal
�
Article history:
Received 27 May 2024
Accepted 7 December 2024
Available online 20 December 2024
�
�
Abstract
Objectives: Biomimetic remineralization strategies offer promising paths for managing caries- affected dentin by restoring its morphology and structural integrity. This pilot study aimed to assess the morphological and structural changes in dentin following two chemical artificial caries-affected dentin (ACAD) protocols, evaluate the effects of three biomimetic remineralization procedures (BRPs) on dentin structure, and determine the most effective ACAD-BRP combination. The BRPs studied were casein phosphopeptide-amorphous calcium phosphate (CPP-ACP), self-assembling P11-4 (SAP-P11-4), and calcium phosphate polymer-induced liquid precursor (Ca/P-PILP).
Methods: Seventy-two specimens of healthy human dentin were divided into twelve groups. Half of each group�s samples were analyzed using field-emission gun environmental scanning electron microscope/energy dispersive X-ray, while the other half were analyzed by X-ray diffraction and micro-Raman spectroscopy.
Results: Results demonstrate alterations in dentin morphology and structure induced by the ACAD protocols, affirming their effectiveness in mimicking caries-affected dentin. Among the BRPs evaluated, CPP-ACP emerged as the most promising agent for promoting remineralization and restoring dentin structure. The combination of pH cycling with CPP-ACP exhibited superior efficacy in promoting dentin remineralization compared to other combinations.
Conclusions: These findings highlight the potential of biomimetic remineralization strategies, particularly CPP-ACP combined with pH cycling, as effective approaches for managing caries-affected dentin and preserving dental health.
Keywords: Biomimetics, Demineralized dentin matrix,Dentin,Tooth remineralization
�
Resumo
Objetivos: As estrat�gias de remineraliza��o biomim�tica oferecem caminhos promissores para a gest�o da dentina afetada por c�rie, restaurando a morfologia e integridade estrutural. Este estudo piloto teve como objetivo avaliar as altera��es morfol�gicas e estruturais na dentina ap�s dois protocolos qu�micos de dentina artificialmente afetada por c�rie (DAAC), avaliar os efeitos de tr�s procedimentos de remineraliza��o biomim�tica (PRB) na estrutura da dentina e determinar a combina��o mais eficaz de protocolo de DAAC e PRB. Os PRB estudados foram fosfopept�deo de case�na-fosfato de c�lcio amorfo (CPP-ACP), p�ptido de automontagem P11-4 (SAP-P11-4) e o indutor do pol�mero precursor l�quido do fosfato de c�lcio (Ca/P-PILP).
M�todos: Dividiram-se 72 amostras de dentina humana saud�vel em doze grupos. Metade das amostras de cada grupo foram analisadas por microsc�pio eletr�nico de varrimento/ raios X de dispers�o de energia, enquanto a outra metade foi analisada por difra��o de raios X e espectroscopia de micro Raman.
Resultados: Os resultados demonstram altera��es na morfologia e estrutura da dentina induzidas pelos protocolos DAAC, confirmando a sua efic�cia em mimetizar esta dentina. Entre os PRB avaliados, o CPP-ACP emergiu como o agente mais promissor para promover a remineraliza��o e restaurar a estrutura da dentina. A combina��o de ciclos de pH com CPP-ACP exibiu efic�cia superior na promo��o da remineraliza��o dentin�ria comparativamente a outras combina��es.
Conclus�es: Estes resultados sublinham o potencial das estrat�gias de remineraliza��o biomim�tica, particularmente CPP-ACP com os ciclos de pH, como abordagens eficazes para o tratamento da dentina afetada pela c�rie e para a preserva��o da sa�de dent�ria.
Palavras-chave: Biomim�tica, Matriz de dentina desmineralizada,Dentina, Remineraliza��o dent�ria
�
Introduction
Dental caries is a disease characterized by the progressive demineralization of dental tissues due to the action of acids produced by bacteria in the oral biofilm.1 While affected enamel may be able to remineralize, dentin is often irreversibly damaged due to the breakdown of collagen bonds and the denaturation of odontoblasts.2
One objective of modern dentistry is to manage caries lesions non-invasively through remineralization, intended to prevent the progression of this disease and improve the resistance, aesthetics, and function of the affected tissues.3 Biomimetic remineralization emerges as a promising response aimed at protecting exposed collagen in demineralized dentin layers by depositing minerals inside and between the fibers, as it occurs naturally, thus restoring structure and mechanical characteristics<4 Remineralizing solutions containing calcium and phosphate have been used to promote the formation of a new remineralized layer on the dentin surface.5 - 7
Among the various biomimetic remineralization procedures (BRP), casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) has stood out for its efficacy in promoting dentin remineralization.5 8 CPP-ACP, a nano-complex of casein protein and amorphous calcium phosphate (ACP), releases and stabilizes high calcium and phosphate ion concentrations on tooth surfaces.9 Self-assembling P11-4 (SAP-P11-4) is another promising agent that promotes remineralization by forming 3D hierarchical structures. SAP-P11-4 binds to calcium ions, causing the precipitation of calcium phosphate salts in the structure, thus facilitating hydroxyapatite nucleation.10 Another approach is the calcium phosphate polymer-induced liquid precursor (Ca/P-PILP), which has shown the potential to effectively promote demineralized dentin remineralization through analogs of non-collagenous proteins.11
Biomimetic remineralization is an important method for restoring the integrity of caries-affected dentin, and diferente options are available. Thus, it would be interesting to check in vitro the effectiveness of biomimetic remineralization with different agents in restoring the integrity of caries-affected dentin previously prepared with different chemical artificial caries-affected dentin (ACAD) protocols.
For that purpose, a pilot study was held with the following objectives: 1) to assess whether there are morphological and structural changes in dentin after two different chemical ACAD protocols; 2) to assess the effects of three different BRPs (CPP-ACP, SAP-P11-4, and Ca/P-PILP) on the dentin structure; 3) to determine the most favorable ACAD-BRP combination. The null hypothesis is that there are no differences in any of these objectives between ACAD and natural dentin. We will use advanced techniques, including field-emission gun environmental scanning electron microscope/energy-dispersive X-ray (FEG ESEM/EDAX), X-ray diffraction (XRD), and micro-Raman spectroscopy (MRS), to analyze the null hypothesis.
Material and Methods
The study was approved by the FMDUP Ethics Committee with the reference �Projeto n� 22/2021.� For this pilot in-vitro study, 18 non-carious permanent human molars extracted for orthodontic or periodontal reasons were selected after clinical examination.
The teeth with visible hypoplastic lesions, crack lines, or detectable carious defects were excluded from the study sample. The teeth were disinfected with 0.5% chloramine-T solution (Merck/Germany) for a week and stored in saline solution at 4�C until the experimental period (ISO/TS 11405) for up to 6 months after the extraction.
Eighteen dentin disks of 1.5-mm thickness were prepared using a hard-tissue microtome (Struers Accutom-5, United States of America (USA)) under running water. The exposed surface was polished with 120-grit silicon carbide sandpaper under running water (Struers Rotopol-11, USA) for 1 minute to simulate the smear layer.5
The dentin disks were then divided into four segments using a Horico diamond disk (Transvident, Germany) under running water. Seventy-two specimens were randomly distributed into 12 groups (Table 1) of N=6: one control group (G1) and 11 intervention groups (G2�G12). The control group in our study consisted of natural dentin, which was used as a baseline to compare the morphological and structural changes observed in the experimental groups. A single operator carried out all protocol procedures (Figure 1) by following the laboratory protocols for each group (Table 2). All groups were then analyzed using FEG ESEM/EDAX, MRS, and XRD.
�
Table 1. Experimental groups

�
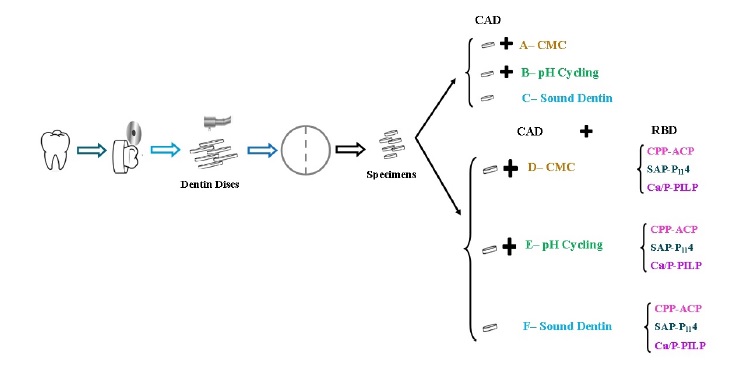
Figure 1. Experimental protocol
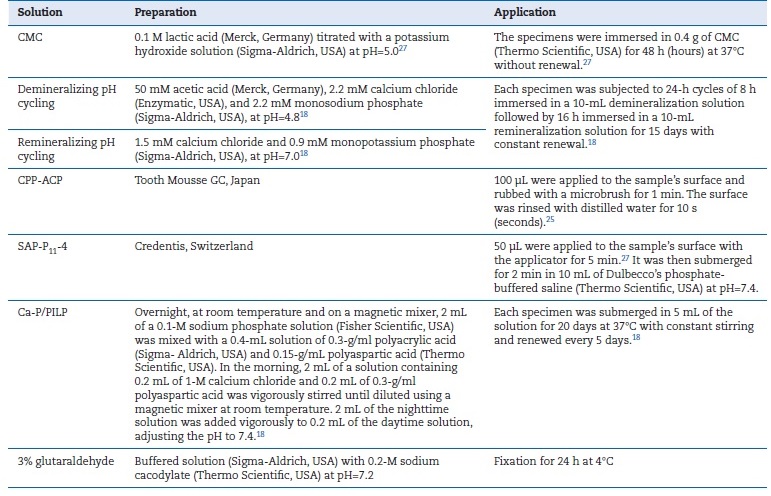
Table 2. Description of laboratory protocols
�
A small groove was created in each specimen with a Diamond disk before fixation in 3% glutaraldehyde for 24h at 4�C. They were then subjected to three consecutive 20-minute baths in sodium cacodylate solution, dehydrated in increasing concentrations of ethyl alcohol (Merck, Germany), and dried with crit-ACAD � artificially caries-affected dentin; BRP � biomimetic remineralization procedures; � � absent; CMC � 6% carboxymethylcellulose acid gel; CPP-ACP � casein phosphopeptide-amorphous calcium phosphate; SAP-P11-4 � self-assembling peptide11-4; Ca/P-PILP � calcium phosphate polymer-induced liquid precursor.
Three samples were used for the FEG ESEM/EDAX analysis.
A small groove was created in each specimen with a Diamond disk before fixation in 3% glutaraldehyde for 24h at 4�C. They were then subjected to three consecutive 20-minute baths in sodium cacodylate solution, dehydrated in increasing concentrations of ethyl alcohol (Merck, Germany), and dried with critical point drying.12 Afterward, the samples were briefly submerged in liquid nitrogen and divided into two fragments by applying pressure to create the cross-section view. These fragments were fixed to SEM stubs with carbon tape and metalized with a thin gold/palladium film by sputtering using the SPI Module Sputter Coater. A blind operator conducted the FEG ESEM/EDAX examination (FEI Quanta 400/Genesis X4M). Qualitative and quantitative analyses were performed: qualitative analysis of microphotographs obtained in FEG ESEM and quantitative analysis based on the average of three specimen measurements obtained by a software coupled with EDAX.
The remaining three samples were analyzed using MRS and XRD. The MRS analysis was conducted using the inVia Raman Microscope (Renishaw, United Kingdom) equipped with a 785-nm red diode laser. Measurements were carried out at room temperature, focusing on three different points on the specimens with a 50x magnifying lens. Acquisition parameters included a range of 200-1800cm-1, an integration time of 10s per scan, the addition of three scans, and a maximum laser power of 115 mW to avoid heat-induced artifacts. Spectra were presented and compared after subtraction of a smoothed baseline and normalization to percentage values using the peak of the most intense Raman mode at around 960 cm-1. Raman mode parameters were calculated using IgorPro software based on the best fit of a sum of damped oscillator functions.
For the XRD analysis, the same samples were ground, prepared, and mounted on a standard sample holder for powders. Measurements were conducted at room temperature using a PANalytical X�Pert Pro diffractometer equipped with an X�Celerator detector and a secondary monochromator in θ/2θ Bragg-Brentano geometry. The measurements used were 40 kV and 30 mA, Cu Kα radiation (λα1 = 1.54060 �;λα2 = 1.54443 �), 0.017�/step, 100 s/step, and 2θ 10-90� angular range. Diffractograms were interpreted (qualitative evaluation), phases were identified using HighScore Plus 4.8 software, and Rietveld refinement of the diffractograms was conducted using the PowderCell 2.4 software. A quantitative analysis using a simple average of three samples was executed to analyze MRS comparative modes and XRD Rietveld analysis.
�
Results
In G2, the FEG ESEM/EDAX analysis revealed no noteworthy changes in inorganic ion concentrations and only slight exposure of the collagen layer within the dentinal tubules. In G7, G8, and G9, where BRPs were performed, no ion concentration variation was observed either. On the other hand, G3 showed a clear change in dentinal tubules�they were widely open and more porous, resulting in demineralization evidenced by decreased levels of inorganic ions calcium and phosphate. G4 had a slight change in the filling of dentinal tubules. G6, G9, and G12 appeared to undergo demineralization and exposure of dentinal tubules. Lastly, G10 and G11 showed an increase in inorganic ions, with G10 showing the most expressive increase in matrix inorganic ions. These results were very similar to those of G1/Control, apart from an abrupt decrease in the percentage of the organic ion carbon (Figure 2).
�
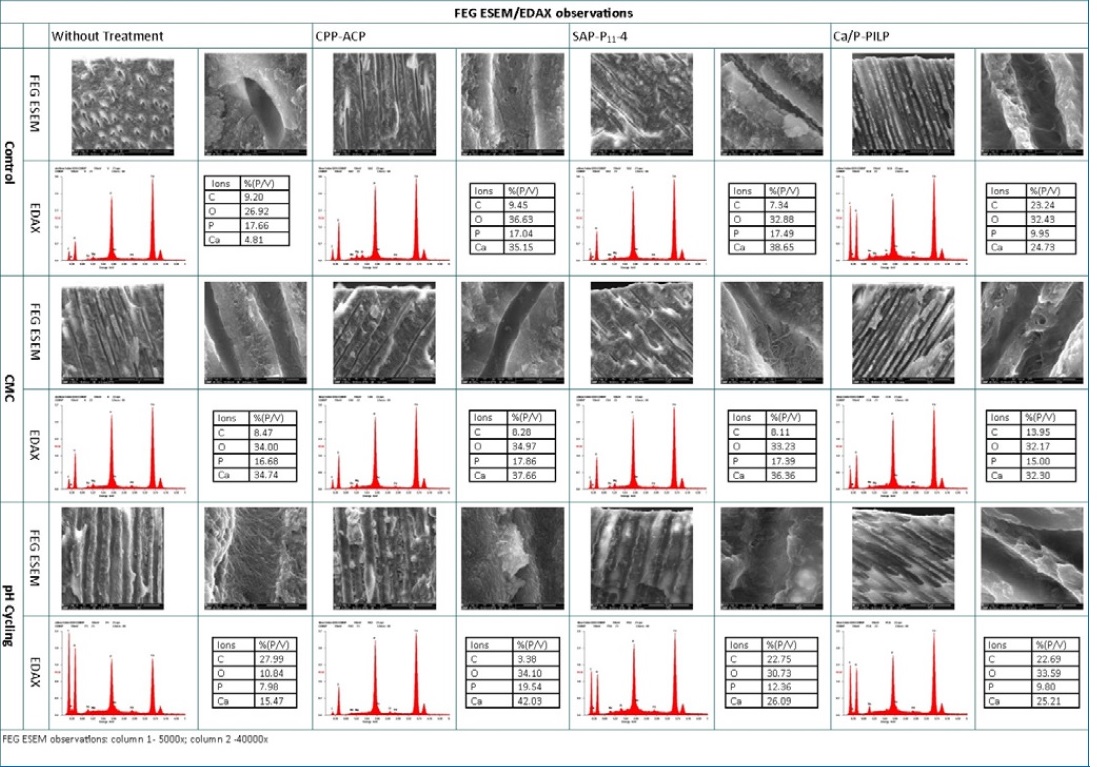
Figure 2. FEG ESEM/EDAX observations
�
The ions� local environment affected each group�s resonance frequency fingerprint, which became sensitive to compositional and structural alterations induced by the protocols.
Compared to the Raman mode spectra from G1/Control, the most evident alterations were detected in G3, G10, G11, and G12. The degradation of dentin quality was evident in G3, reducing the efficiency of Raman emission from the sample surface, leading to noisier spectra, and affecting mode profiles.
The 10�20% increase in intensity ratios of modes associated with collagen organic groups suggests a substantial decrease in the quantity and quality of the mineral dentin component. G10�s procedure caused the most positive alterations in the dentin (Figure 3-A). Comparing the XRD spectra obtained under the same acquisition parameters, G1/Control, G4, G6, and G10 showed sharpening, deconvolution, and discretization of diffraction peaks. On the other hand, G2, G3, G5, and G12 suggested some contraction of the cell lattice volume and possibly degradation of crystallization quality (Figure 3-B).
�
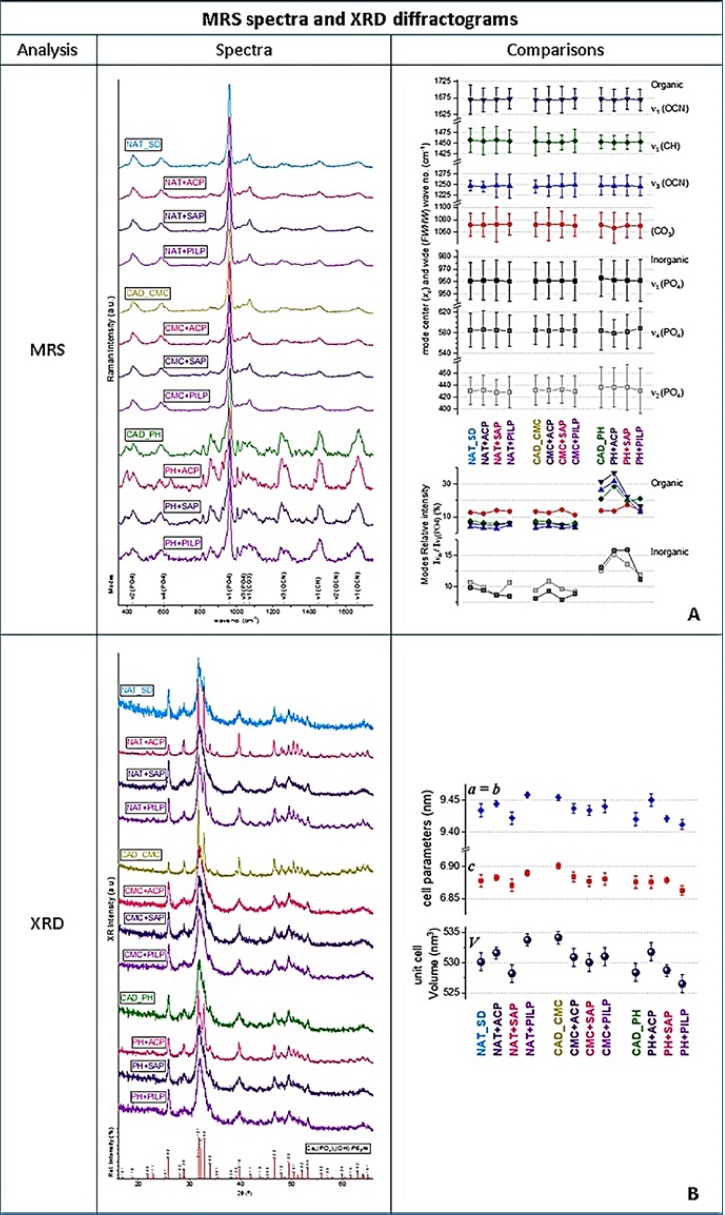
Figure 3. MRS spectra and XRD diffractograms. �A. Comparison of representative Roman module parameters in the spectral region between 300 and 1750 cm-1. A simplified interpretation was based on the intensity/dimension of some modules� peaks as an indication of the presence/absence of the oscillator that originates the module34,35, i.e., phosphate (PO4)3- (u2 at 400�470 cm4, u4 at 520�600 cm-1, and u1 and u3 at 960-1100 cm-1), and modes related to the organic radicals: carbonate (CO3)2 at ~1070 cm-1, carboxyl (CH) at ~1450 cm-1, and amide (OCN) at 1240�1270 cm-1, 1500�1550 cm-1, and 1600�1700 cm-1. B. Structural parameters of the hydroxyapatite componente obtained by a detailed Rietveld analysis.15 The indexation was made to the polycrystalline phase of the hexagonal space group P63/m (176) and composed of two formula units, with formula Ca5(PO4)3(OH), according to reference.36,37�
Discussion
Biomimetic remineralization has emerged as a promising strategy to reverse the adverse effects of caries lesions by restoring the structure and mechanical properties of demineralized dentin.13 Our pilot study aimed to assess morphological and structural changes in dentin following two chemical ACAD protocols, evaluate the effects of three BRPs�CPP-ACP, SAP-P11-4, and Ca/P-PILP�on dentin structure, and determine the most favorable trend of the ACAD-BRP combination.
While XRD diffractograms are mainly correlated to the long-range mineral lattice order, MSR spectroscopy can be sensitive to dentin�s inorganic and organic constituents. MRS studies the assembly of allowed vibration modes characteristic of a molecule/structural frame that defines an active Raman oscillator. Generally, a relative change occurring in such structures will translate into the respective Raman modes, varying in peak center (xc) and width (FWHW) compared to the reference mode. A major narrowing of peaks corresponds to a decrease in structural disorder, while an increase in mode wavenumber can correspond to a rigid oscillator related to stronger or less flexible molecular bonds.14, 15
The sharpening, deconvolution, and discretization of diffraction peaks in XRD diffractograms correlated by Scherrer16 result from a notable growth in crystallite average size, decreasing the contribution to lattice distortions of grain boundaries.
These XRD Rietveld analyses reveal important recrystallization with a lattice volume expansion of +0.3 to +0.8% and an improvement in the quality of hydroxyapatite crystallization. Generally, these losses of inorganic ions, or degradation of hydrogen bridges between molecules, promote repulsion in the framework of anionic oxygen (a practical indication of dentin microstructure regeneration).
The results of our study reject the null hypothesis, demonstrating clear morphological and structural differences compared to natural dentin. Both chemical ACAD protocols increased surface demineralization and altered dentin morphology and structure. The literature suggests that pH cycling is more effective than acidified gel because it produces a thicker layer of demineralization. Our findings agree that pH cycling might be more appropriate than other procedures to simulate a substrate after a caries lesion.17 This chemical model effectively reproduced the dynamic processes involved in caries progression, closely mimicking in-vivo demineralization conditions. 18
While both Ca/P-PILP and SAP-P11-4 demonstrated efficacy in altering dentin mineral composition, they did not achieve the same level of efficacy as CPP-ACP in promoting remineralization.
According to the literature, P11-4 can interact with type-I collagen, boosting the hybrid layer�s stability and the fibers� resilience to proteolysis while supporting mineral development.19 The functional domain of natural proteins controlling the initiation and progression of the biomimetic remineralization process in Ca/P-PILP can be replicated by non-collagenous-protein analogs with high affinities for Ca2+ and collagen.18, 20 These analogs also act as inhibitors of ACP nanoparticle aggregation.21
Furthermore, combining CPP-ACP with pH cycling showed superior remineralization effects, agreeing with previous literature.9, 22, 24 CPP-ACP exhibits synergistic effects in promoting dentin remineralization, as the acidic environment during demineralization facilitates the release of calcium and phosphate ions from CPP-ACP.25 26 These ions can then be deposited onto the demineralized tooth surface during remineralization, promoting hydroxyapatite crystal formation.24, 27 Studies have consistently demonstrated that the combination of CPP-ACP and pH cycling leads to increased mineral uptake and improved remineralization of dentin lesions compared to either treatment alone. Additionally, CPPACP has been found to inhibit cariogenic bacteria�s growth and reduce caries lesions� progression, emphasizing its value as na adjunct in managing caries-affected dentin.22, 24
Our results suggest that CPP-ACP may offer a clinically viable approach to managing caries-affected dentin. By effectively reversing the demineralization process and promoting remineralization, CPP-ACP has the potential to arrest caries progression, thereby preserving dentin structure and function.25
This study aimed to assess the trend toward a favorable ACAD-BRP combination. It should be noted that this is a preliminary study with a consequently small sample size. Therefore, the limitations of this pilot in-vitro study should be acknowledged, and the interpretation of the results should be cautious. Further studies with larger sample sizes are warranted to validate our findings.28 - 37 Additionally, future research should consider varying the experimental conditions, such as different pH levels, times or temperatures, to better understand the full potential and limitations of these biomimetic remineralization protocols in diverse clinical scenarios.
Nevertheless, our study highlights the potential of CPP-ACP as a valuable adjuvant in caries lesion remineralization and the importance of considering dynamic environmental factors, such as pH fluctuations, in experimental models evaluating BRP.
Conclusions
The results show changes in dentin morphology and structure, confirming the potential effectiveness of the two ACAD chemical protocols in inducing changes in dentin, with the pH cycling protocol being more promising. Different degrees of efficacy were found between the BRPs, with CPP-ACP emerging as the most promising agent for promoting remineralization and restoring dentin structure in both ACADs. The combination of pH cycles with CPP-ACP showed a superior ability to promote dentin remineralization compared to the other combinations evaluated in this in-vitro study.
�
References
1. Kidd EAM. Clinical threshold for carious tissue removal. Dent Clin North Am. 2010;54:541-9.
2. Deyhle H, Bunk O, M�ller B. Nanostructure of healthy and caries-affected human teeth. Nanomedicine. 2011;7:694�701.
3. Dawasaz AA, Togo RA, Mahmood Z, Ahmad A, Ponnuraj KT. Remineralization of Dentinal Lesions Using Biomimetic Agents: A Systematic Review and Meta-Analysis. Biomimetics (Basel). 2023;8:159.
4. Mai S, Kim YK, Toledano M, Breschi L, Ling JQ, Pashley DH, et al. Phosphoric acid esters cannot replace polyvinylphosphonic acid as phosphoprotein analogs in biomimetic remineralization of resin-bonded dentin. Dent Mater. 2009;25:1230-9.
5. Barbosa-Martins LF, Sousa JP, Alves LA, Davies RPW, Puppin- Rontanti RM. Biomimetic Mineralizing Agents Recover the Micro Tensile Bond Strength of Demineralized Dentin. Materials (Basel).2018;11:1733.
6. Braga RR, Fronza BM. The use of bioactive particles and biomimetic analogues for increasing the longevity of resin-dentin interfaces: A literature review. Dent Mater J. 2020;39:62-8.
7. Gandolfi MG, Taddei P, Siboni F, Modena E, De Stefano ED, Prati C. Biomimetic remineralization of human dentin using promising innovative calciumsilicate hybrid �smart� materials. Dent Mater. 2011;27:1055�69.
8. Li Y, Chen X, Fok A, Rodriguez-Cabello JC, Aparicio C. Biomimetic Mineralization of Recombinamer-Based Hydrogels toward Controlled Morphologies and High Mineral Density. ACS Appl Mater Interfaces. 2015;7:25784-92.
9. Poggio C, Lombardini M, Vigorelli P, Ceci M. Analysis of dentin/enamel remineralization by a CPP-ACP paste: AFM and SEM study. Scanning. 2013;35:366-74.
10. Kirkham J, Firth A, Vernals D, Boden N, Robinson C, Shore RC, et al. Self-assembling peptide scaffolds promote enamel remineralization. J Dent Res. 2007;86:426-30.
11. Sear RP. The non-classical nucleation of crystals: microscopic mechanisms and applications to molecular crystals, ice and calcium carbonate. International Materials Reviews. 2012;57:328-56.
12. Bray D. Critical Point Drying of Biological Specimens for Scanning Electron Microscopy. In: Williams JR, Clifford AA editors. Supercritical Fluid Methods and Protocols. Methods in Biotechnology. 13th ed. Humana Press, 2008.
13. Bacino M, Girn V, Nurrohman H, Saeki K, Marshall SJ, Gower L, et al. Integrating the PILP-mineralization process into a restorative dental treatment. Dent Mater. 2019;35:53-63.
14. Adachi T, Pezzotti, G, Yamamoto T, Ichioka H, Boffelli M, Zhu W, et al. Vibrational algorithms for quantitative crystallographic analyses of hydroxyapatite-based biomaterials: II, application to decayed human teeth. Anal Bioanal Chem. 2015;407: 3343�56.
15. Pezzotti G, Zhu W, Boffelli M, Adachi T, Ichioka H, Yamamoto T, et al. Vibrational algorithms for quantitative crystallographic analyses of hydroxyapatite-based biomaterials: I, theoretical foundations. Anal Bioanal Chem. 2015;407:3325�42.
16. Cullity BD, Stock SR editors. Elements of X-Ray Diffraction. 3rd ed. Essex: Pearson Education Limited, 2014.
17. Marquezan M, Correa FNP, Sanabe ME, Rodrigues Filho LE, Hebling J, Guedes-Pinto AC, et al. Artificial methods of dentine caries induction: A hardness and morphological comparative study. Arch Oral Biol. 2009;54:1111-7.
18. Chen R, Jin R, Li X, Fang X, Yuan D, Chen Z, et al. Biomimetic remineralization of artificial caries dentin lesion using Ca/P-PILP. Dent Mater. 2020;36:1397-1406.
19. de Sousa JP, Carvalho RG, Barbosa-Martins LF, Torquato RJS, Mugnol KCU, Nascimento FD, et al. The Self-Assembling Peptide P11-4 Prevents Collagen Proteolysis in Dentin. J Dent Res. 2019;98:347-54.
20. Niu LN, Zhang W, Pashley DH, Breschi L, Mao J, Chen JH, et al. Biomimetic remineralization of dentin. Dent Mater. 2014;30:77-96.
21. Gower LB. Biomimetic model systems for investigating the amorphous precursor pathway and its role in biomineralization. Chem Rev. 2008;108:4551�627.
22. Rahiotis C, Vougiouklakis G. Effect of a CPP-ACP agent on the demineralization and remineralization of dentine in vitro. J Dent. 2007;35:695-8.
23. Adebayo OA, Burrow MF, Tyas MJ. Resin-dentine interfacial morphology following CPP-ACP treatment. J Dent. 2010;38:96-105.
24. Cao Y, Mei ML, Xu J, Lo ECM, Li Q, Chu CH. Biomimetic mineralisation of phosphorylated dentine by CPP-ACP. J Dent. 2013;41:818-25.
25. Moreira KM, Bertassoni LE, Davies RP, Joia F, Hofling JF, Nascimento FD, et al. Impact of biomineralization on resin/ biomineralized dentin bond longevity in a minimally invasive approach: An �in vitro� 18-month follow-up. Dent Mater. 2021;37:e276-89.
26. Pulidindi H, Mandava J, Borugadda R, Ravi R, Angadala P, Penmatsa P. Effect of remineralizing agents on resin-dentin bond durability of adhesive restorations: An in vitro study. J Int Oral Health. 2021;13:470-7.
27. Barbosa-Martins LF, de Sousa JP, de Castilho ARF, Puppin- Rontani J, Davies RPW, Puppin-Rontani RM. Enhancing bond strength on demineralized dentin by pre-treatment with selective remineralising agents. J Mech Behav Biomed Mater. 2018;81:214-21.
28. Rueggeberg FA. Substrate for adhesion testing to tooth structure: review of the literature. Dent Mater. 1991;7:2-10.
29. S�derholm KJ. Correlation of in vivo and in vitro performance of adhesive restorative materials: a report of the ASC MD156 Task Group on test methods for the adhesion of restorative materials. Dent Mater. 1991;7:74-83.
30. Armstrong SR, Boyer DB, Keller JC. Microtensile bond strength testing and failure analysis of two dentin adhesives. Dent Mater. 1998;14:44-50.
31. Pashley DH. Dentin bonding: overview of the substrate with respect to adhesive material. J Esthet Dent. 1991;3:46-50.
32. Sirisha K, Rambabu T, Ravishankar Y, Ravikumar P. Validity of bond strength tests: A critical review-Part II. J Conserv Dent. 2014;17:420-6.10.4103/0972-0707.139823
33. Sirisha K, Rambabu T, Ravishankar Y, Ravikumar P. Validity of bond strength tests: A critical review: Part I. J Conserv Dent. 2014;17:305-11.
34. Marin E, Hiraishi N, Honma T, Boschetto F, Zanocco M, Zhu W, et al. Raman spectroscopy for early detection andmonitoring of dentin demineralization. Dent Mater. 2020;36:1635-44.
35. Tian F, Zhou L, Zhang Z, Niu L, Zhang L, Chen C, et al. Paucity of Nanolayering in Resin-Dentin Interfaces of MDP-based Adhesives. J Dent Res. 2016;95:380-7.
36. Hanlie H, Liyun T, Tao J. The Crystal Characteristics of Enamel and Dentin by XRD Method. J Wuhan Univ Technol � Mater Sci Ed. 2006;21:9-12.
37. Rodr�guez-Lorenzo LM, Hart JN, Gross KA. Structural and Chemical Analysis of Well-Crystallized Hydroxyfluorapatites. J Phys Chem B. 2003;107:8316�20.
�
Ros�rio Costa
E-mail address: mrcosta@fmd.up.pt
�
Conflict of interest
The authors have no conflicts of interest to declare.
�
Ethical disclosures
Protection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of data. The authors declare that no patient data appear in this article.
Right to privacy and informed consent. The authors declare that no patient data appear in this article.
�
CRediT authorship contribution statement
Ros�rio Costa: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing � original draft. Sofia Arantes-Oliveira: Conceptualization, Methodology, Validation, Supervision, Writing � review & editing. Lisete Fernandes: Data curation, Formal analysis. F�bio G. Figueiras: Data curation, Formal analysis, Writing � original draft. Jo�o Cardoso Ferreira: Conceptualization, Validation, Supervision, Writing � review & editing. Paulo Ribeiro de Melo: Conceptualization, Methodology, Validation, Supervision, Project administration, Writing � review & editing.
�
Acknowledgements
The authors would like to thank the Bone Lab of the Pharmacy Department, Faculty of Dental Medicine of the University of Porto, for their valuable collaboration in laboratory protocols, the Electron Microscopy Unit, University of Tr�s-os-Montes and Alto Douro, for the X-ray diffraction analysis, and the Department of Physics and Astronomy, Faculty of Sciences of the University of Porto, for the micro Raman spectroscopy equipment.
�
1646-2890/� 2024 Sociedade Portuguesa de Estomatologia e Medicina Dent�ria. Published by SPEMD.
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
