
Revista Portuguesa de Estomatologia, Medicina Dentária e Cirurgia Maxilofacial
SPEMD - Revista Portuguesa de Estomatologia Medicina Dentária e Cirurgia Maxilofacial | 2024 | 65 (3) | 121-128
Original research
Effects of irrigation activation techniques on root canal walls after post-space preparation: A scanning electron microscopy study
Efeitos de técnicas de ativação de irrigação na parede do canal radicular após a preparação para espigão: um estudo com microscopia eletrónica de varrimento
a Department of Endodontics, Faculty of Dentistry, Akdeniz University, Antalya, Turkey
b Electron Microscopy Image Analyzing Unit and Department of Biophysics, Faculty of Medicine, Akdeniz University, Antalya, Turkey
Kür?at Er - qursater@hotmail.com
Article Info
Rev Port Estomatol Med Dent Cir Maxilofac
Volume - 65
Issue - 3
Original research
Pages - 121-128
Go to Volume
Article History
Received on 10/11/2023
Accepted on 07/09/2024
Available Online on 30/09/2024
Keywords
Original Research
�
Effects of irrigation activation techniques on root canal walls after post-space preparation: A scanning electron microscopy study
Efeitos de t�cnicas de ativa��o de irriga��o na parede do canal radicular ap�s a prepara��o para espig�o: um estudo com microscopia eletr�nica de varrimento
�
Dide Tekinarslan1 0000-0002-7818-4992
Damla Erkal1 0000-0001-8319-6974
Simay Ko�1 0000-0002-9446-5655
Hakan Er2 0000-0001-7739-4712
K�rşat Er1,* 0000-0002-0667-4909
1 Department of Endodontics, Faculty of Dentistry, Akdeniz University, Antalya, Turkey
2 Electron Microscopy Image Analyzing Unit and Department of Biophysics, Faculty of Medicine, Akdeniz University, Antalya, Turkey
�
�
Article history:
Received 10 November 2023
Accepted 7 September 2024
Available online 30 September 2024
�
Abstract
Objectives: To evaluate the effect of different irrigation activation techniques (IATs) on smear layer removal after post-space preparation.
Methods: Forty single-rooted mandibular premolars with no root canal treatment were selected. Teeth were decoronated at 15 mm, and apical patency was confirmed using a #10 K-file. The ProTaper Next NiTi file system was used to shape the root canal. Afterward, the root canals were filled with gutta-percha and a root canal sealer. A week later, 10-mm postspace preparation was done. Teeth were randomly divided into 4 groups based on the IATs used to activate 3 mL of 17% EDTA for 1 min: 1) control group with a conventional needle; 2) sonic irrigation (SI); 3) passive ultrasonic irrigation (PUI); and 4) laser-activated irrigation (LAI). Teeth were split longitudinally, and specimens were observed under a scanning electron microscope. Images were taken at the teeth� coronal, middle, and apical thirds at �2000 magnification and were scored based on the presence of the smear layer. Data were analyzed statistically.
Results: No statistically significant difference was observed between the IATs regarding smear layer removal (p>0.05). However, PUI was more successful at the coronal and apical third, and SI was more successful in the middle third.
Conclusions: All the used IATs were effective in removing the smear layer. However, none of them removed it completely
Keywords: Irrigation activation, Post-space preparation,Scanning electron microscopy,Smear layer
�
Resumo
Objetivos: Avaliar o efeito de t�cnicas de ativa��o de irriga��o na remo��o da smear layer ap�s a prepara��o do espa�o para espig�o.
M�todos: Selecionaram-se 40 pr�-molares mandibulares de raiz �nica sem tratamento endod�ntico. Removeu-se a coroa dos dentes aos 15 mm, e confirmou-se a permeabilidade apical com uma lima K #10. O sistema de limas NiTi ProTaper Next foi utilizado para a prepara��o canalar, e os canais foram obturados com guta-percha e cimento. Uma semana depois, preparou-se o espa�o de 10 mm para espig�o. Os dentes foram divididos aleatoriamente em 4 grupos com base na t�cnica de ativa��o de irriga��o utilizada para ativar 3 mL de EDTA a 17% durante 1 min: 1) grupo de controlo com agulha convencional; 2) irriga��o s�nica (SI); 3) irriga��o ultrass�nica passiva (PUI); e 4) irriga��o ativada a laser (LAI), e observados sob um microsc�pio eletr�nico de varrimento. Analisaram-se imagens dos ter�os coron�rio, m�dio e apical com amplia��o de �2000, e documentou-se a presen�a da smear layer. Os dados foram analisados estatisticamente.
Resultados: N�o foi observada diferen�a estatisticamente significativa entre as t�cnicas de ativa��o de irriga��o em termos de remo��o da smear layer (p>0.05). Por outro lado, a PUI foi mais eficaz no ter�o coron�rio e apical, enquanto a SI teve melhor desempenho no ter�o m�dio
Conclus�es: Todas as t�cnicas de ativa��o de irriga��o utilizadas mostraram-se eficazes na remo��o da smear layer. No entanto, nenhuma das t�cnicas foi capaz de a remover totalmente.
Palavras-chave: Ativa��o de irriga��o, Prepara��o de espa�o para espig�o, Microscopia eletr�nica de varrimento, Smear layer
�
Introduction
Coronal restoration of teeth following root canal treatment generally requires additional support from the root canal system, which endodontic post systems can provide.1 Fiber-reinforced posts have a significant proportion of fibers inside a polymer matrix that constructs highly cross-linked structures.2 Recent studies2 3 showed that post systems should have equal or similar rigidity to the tooth to transmit occlusal forces equally on root surfaces. Nowadays, fiber-reinforced posts used with advanced adhesive systems are a great restorative option because of their high aesthetic properties, flexibility, and similar elasticity to dentin.3
Adequate bonding among the fiber post, dentin, and adhesive cement is important and provides increased restoration stability.4 The bonding effectiveness depends on the smear layer (SL) removal and the adhesive system.5 After preparing the fiber post, the SL covers the dentin tubules, restraining the bonding of adhesive cements and sealers.6 The SL consists of organic and inorganic particles and bacterial products, which promote infection persistence and decreased intratubular dentin penetration.7
Thus, the SL removal enhances the penetration of sealers int dentin tubules and facilitates root canal cleaning.7 Various chemical substances can be used to modify the hybrid layer�s longevity and decrease its breakdown, with collagen cross-linkers, protease inhibitors, and enzyme inhibitors being the primary agents. These techniques are well described in the literature.9 10 The elements of the targeted SL dictate these medications� modes of action. Deproteinizing compounds can be used to break down the organic componentes of the SL, while chelating agents can bind to the inorganic components.11, 12 Furthermore, SL removal can be enhanced by hand-adjusting the application of adhesive cement. 13
Current methods of SL removal depend on chemical irrigation, irrigation activation techniques (IATs), and their combination.14 Some IATs, such as manual, sonic, ultrasonic, and laser-activated techniques, get irrigation solutions in contact with the whole root canal system for effective action.15 When using a needle for traditional irrigation, only the tip of the needle is in contact, which can lead to less disinfection activity.7 Sonic irrigation (SI) produces mechanical oscillation largely at the tip of the file. In contrast, passive ultrasonic irrigation (PUI) systems produce acoustic waves and combine them with the chemical action of the irrigation along the noncutting file.16 This microstreaming action moves the irrigation agente along the surface of the canals and enhances mechanical cleaning.17 Some studies have shown that activation with sonic and ultrasonic systems provides cleaner surfaces than traditional irrigation with a needle.18 19,
Laser-activated irrigation (LAI) is an IAT using medium-infrared lasers (2780 and 2940 nm) that has emerged as a supplementary technique for irrigating root canals. Water-based solutions strongly absorb the radiation emitted by the laser, causing the formation of inflated and collapsed vapor bubbles at the fiber tip � a process known as cavitation. Variations in collapsed bubble dimensions result in localized shock waves and different fluid motions. Consequently, further cavitation bubbles are induced by succeeding laser pulses. Thus, the irrigant creates acoustic streaming that flows throughout the entire root canal system.15 Er:YAG and Er,Cr:YSGG lasers show great potential as methods for stimulating irrigants. According to Aldeen et al.,20 using Er:YAG laser resulted in a much higher debris removal than PUI and traditional irrigation.
PIPS (photon-induced photoacoustic streaming) is an LAI technique that uses a pulsed Er:YAG laser. This technique employs low pulse energies (10 or 20 mJ) and a short pulse length (50 μs), which leads to high peak powers and effective cavitation.21 This method differs from previous laser IATs by inserting only the tip into the pulp chamber, thus avoiding any contact with the root canal wall. It has been shown to produce a mass of photoacoustic shockwaves capable of spreading the irrigation solutions into dentinal tubules, removing the SL on the dentinal walls.22 Since the development of lasers, PIPS has been used to disinfect the root canal space and remove the SL and other foreign matters.23 PIPS is one of the emerging LAI techniques since 2012. It has lower energy and short pulse length compared to the other LAI methods, thus decreasing thermal damage to the root canals and periodontal tissues.24 Shock wave-enhanced emission photoacoustic streaming (SWEEPS) was developed to enhance the debridement efficiency of the PIPS method. 25 When the cavitation bubble starts to collapse, a secondary pulse is transmitted through the liquid, causing the formation of a second cavitation bubble. This second cavitation bubble speeds up the collapse of the first one, resulting in a powerful collapse and finally releasing a shock wave.26 However, there is very little data about SL removal with LAI systems compared to ultrasonic and sonic activation systems.27, 28
The aim of this in vitro study was to compare the diferente IATs (sonic, ultrasonic, and laser-activated) for their ability to remove SL after post-space preparation. The null hypothesis tested was that there are no differences in efficiency between the IATs used in this study in removing the SL after the postspace preparation.
Material and methods
According to the ANOVA test estimation, with a 0.05 margin of error and a 0.7 power, the minimum number of samples for the study was 36 in total. Since the nonparametric test would be used, 10% was added, and the total number of samples became n = 40.29
This study�s sample consisted of 40 mandibular premolars with a single root. These teeth were extracted due to orthodontic or periodontal issues and had not undergone root canal treatment. Teeth of comparable lengths were selected. Afterward, periapical radiographs were obtained to verify the presence of single canals in the teeth. Teeth with excessively oval anatomy and more than one root canal were excluded.
Teeth with fractures and resorption, as assessed with a stereomicroscope, were also excluded. Residual periodontal tissues and calculus were removed from root surfaces with curettes, and the teeth were preserved in a sterile saline solution until their use.
After measuring working lengths using a stereomicroscope (Zeiss Stemi, Jena, Germany), the teeth were cut to a standardized length of 15 mm. Apical patency was verified using a #10 K-file. Premolars were prepared with ProTaper Next X1, X2, and X3 (Dentsply, Ballaigues, Switzerland), and root canals were irrigated between each file with 2 mL of 2.5% NaOCl using a 30 G side-vented needle attached to a plastic syringe (Canal Clean; Biodent, Paju, South Korea) placed 1-mm short of the working length. Before filling, a final irrigation with 2 mL of 2.5% NaOCl, 2 mL of 17% EDTA, and 2 mL of 2% chlorhexidine, alternated with a saline solution rinse between irrigants, was performed. Subsequently, the teeth were dried using sterile paper points (ProTaper Next X3; Dentsply Sirona, Ballaigues, Switzerland) and filled by the single cone technique using X3 (tip 30, 0.04 taper), gutta-percha (Dentsply, Ballaigues, Switzerland), and an epoxy-resin root canal sealer (Diadent Dia Proseal, Seoul, Korea). After the root canal obturation, access cavities were closed with Teflon tape and Cavit (3M Cavit, Minnesota, USA). Then, the teeth were immersed in distilled water to let the resin sealer (Diadent Dia Proseal, Seoul, Korea) polymerize, keeping the teeth in a moist environment without bacterial contamination. A week later, a 10-mm post-space preparation was done using 1.2-mm and 1.4-mm fiber post drills (Cytec, Hahnenkratt, Germany). Between each drill, teeth were rinsed with 2 mL of saline solution.
After this procedure, the samples were randomly divided into four groups for the final irrigation with the corresponding IAT:
� Group 1 (control group, no activation): Final irrigation was done with a conventional needle (2.5 cc, 27 G) with 3 mL of 17% EDTA for 1 min. Then, the teeth were rinsed with a 5-mL sterile saline solution.
� Group 2 (SI group): Final irrigation activation was done with an SI system (Endo Activator, Dentsply, Santa Barbara, USA) with 3 mL of 17% EDTA at 10,000 cycles per minute using a medium polymer tip (#25/.04) positioned at the post space and activated for 1 min. Then, the teeth were rinsed with a 5-mL sterile saline solution.
� Group 3 (PUI group): Final irrigation activation was done with a PUI system (Satelec Acteon, Merignac, France) with 3 mL of 17% EDTA for 1 min. The PUI system consisted of a noncutting #25 file (Irrisafe; Satelec Acteon, Merignac, France) driven by an ultrasonic device (Satalec P5 Newtron XS; Satelec Acteon, Merignac, France). Ultrasonic activation was done for 3 � 20 sec at 50% power for 1 min, with the tip immersed in the root canal containing irrigant throughout the post space. Then, the teeth were rinsed with a 5-mL sterile saline solution.
� Group 4 (LAI group): Final irrigation activation was done with an LAI device (Waterlase iPlus Biolase, Irvine, USA) with 3 mL of 17% EDTA for 1 min. The laser device was operated at a wavelength of 2780 nm, a panel setting of 0.5 W, and a frequency of 20 Hz, resulting in an energy output of 25 mJ per pulse. No air or water spray was employed during the procedure. The pulses were concentrated using a fiber tip (RFPT5) with a diameter of 580 μm. Then, the teeth were rinsed with a 5-mL sterile saline solution.
After the final irrigation procedures, the teeth were cut off buccolingually into two pieces. Then, all the teeth were dried and stabilized on the aluminum base. Teeth were covered twice with a 20-nm gold-aluminum layer and examined apically from the coronal reference under backscattered electron scanning microscopy (SEM) at 2000x magnification. Their SL removal was graded as follows:30 (0) all dentin tubules can be seen, there is no SL; (1) some of the dentin tubules are open, and some of the dentin tubules are covered with SL; (2) all dentin tubules are covered with SL. In previous studies,31,32 the area examined was described as the coronal, middle, or apical third of the post space, so we divided the post-space cavity into those three sections to compare the results with these previous studies.
The images obtained from the SEM were assessed, and two professionals with expertise in endodontics rated the SL removal.
The inter-operator reliability for these ratings was high, with a Pearson correlation coefficient of 0.994. Two independente and experienced examiners scored the SL removal, and if no consensus was reached, a third independent examiner was involved.
The Kruskal-Wallis H test was employed to compare the four groups, while the Mann-Whitney U test and Bonferroni correction were used for pairwise comparisons. The Friedman test was employed to compare the three post-space regions, while Wilcoxon�s signed rank test and Bonferroni correction were applied for pairwise comparisons. The statistical software SPSS 23.0 (SPSS, Chicago, IL, USA) was used, and a p-value below 5% was considered statistically significant.
Results
In this study, 40 mandibular premolars with post-space preparation were irrigated with four different IATs, whose efficiency in SL removal was evaluated under SEM (Figure 1). Table 1 details the mean and standard deviation values of SL removal scores for the used techniques. The PUI and control group performed superiorly in the coronal third compared to the middle and apical thirds. In turn, LAI was more successful in the middle and coronal thirds than in the apical third. SI achieved higher levels of success in the middle third of the post space compared to other regions. However, there was no statistically significant difference between the IATs (p>0.05). There was also no significant difference in SL removal for each IAT between the post-space apical, middle, and coronal thirds.
�
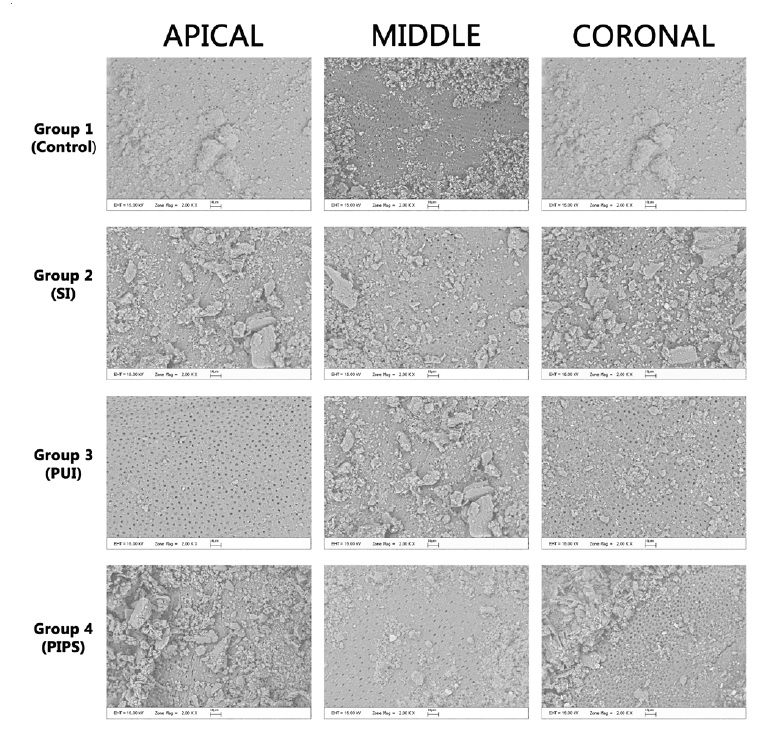
Figure 1. Representative electron scanning microscopy (SEM) images of the smear layer removal by irrigation protocols after post-space preparation.
�
Table 1. Mean and standard deviation values of smear layer removal scores in each irrigation activation technique group per post-space third.
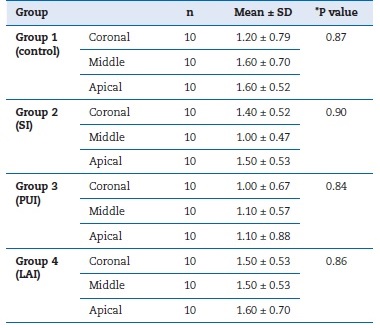
*P value indicated the difference among each group�s coronal, middle, and apical parts. SI � sonic irrigation; PUI � passive ultrasonic irrigation; LAI � laser-activated irrigation.
�
Discussion
The adoption of fiber post restorations has grown over time because of its great aesthetics and dentin-like elastic modulus.3 Adhesive cementation allows passively positioning the post system into the root canals.48 The success of adhesive bonding to dentin in endodontically treated teeth repaired by fiber posts and resin luting systems relies on the establishment of micromechanical retention through a demineralized surface and resin tag formation.33 Optimal adherence requires penetration into dentinal tubules, promoting a stronger bond between the tooth structure and the restorative material.34
The SL is an uneven layer developed during the mechanical preparation of post systems.14 In order to achieve successful dentinal adhesion, the SL should be removed either fully or partially.35 Previous studies8, 36 recommended removing the SL to improve irrigants� antibacterial activity, decrease the reinfection risk, and improve the penetration of sealers, adhesives, and intracanal medications. SL removal is accomplished by irrigants capable of dissolving organic and inorganic layers.14
Sodium hypochlorite (NaOCl) is a flexible antimicrobial solution frequently employed to destroy the biofilms of diferente microorganisms during endodontic treatments.37 Hard tissue deproteinization is another option.38 Research has shown that NaOCl modifies the material�s mechanical characteristics by dissolving the organic components of dentin and that 0.5% NaOCl is less effective on dentin deproteinization than concentrations of 1.0% and 2.25%. However, thorough research is lacking regarding the specific quantitative impacts of low doses (0.5�2.25%) and brief exposure periods (1�10 minutes) of NaOCl.39 Furthermore, the binding strength between dentin surfaces and fiber posts has been demonstrated to decrease after 10 minutes of exposure to 5.0% NaOCl.40
In turn, the prolonged use of EDTA in root canal treatments may contribute to root canal resorption because of its demineralizing impact on root dentin.41 EDTA strengthens the binding between endodontic sealers and dentin by exposing amino groups to demineralization aided by strong decalcifying agents.
Nonetheless, there is a significant danger of weakening the link and increasing interfacial deterioration42due to the sealer�s inadequate penetration into the demineralized dentin, mostly derived from the severe decalcification of the root canal walls.
Furthermore, chelating chemicals like EDTA can modify additional dentin qualities, including micro and nanohardness.43 While removing the SL, irrigants must be in direct contact with canal walls for effective disinfection.7 Chow et al.44 reported that the efficacy of irrigation was related to needle depth. Munoz & Camacho-Cuadra45 showed that irrigants reached no more than 0-1.1 mm beyond the needle tip with conventional needle irrigation, offering decreased disinfection of the complete root canals. Moreover, conventional needle irrigation is less effective in reaching complicated anatomies like anastomoses, isthmus, and lateral canals.6 Shahravan et al.46 stated that removing the SL increases cleanliness and provides tight root canal filling. For this reason, different irrigant activation and delivery devices have been recommended to increase the distribution and effect of irrigants.32
Previous studies40 47 reported that IATs like sonic or ultrasonic may promote better disinfection efficacy by irrigants than conventional irrigation. However, Silva et al.49 reported no significant difference between conventional irrigation and PUI. PUI creates acoustic streaming and cavitation with noncutting action to remove the SL and disinfect the root canals.
Compared to ultrasonic energy, sonic activation has low-frequency vibration and flexible tips that do not deform canal walls as metal ultrasonic activation devices do.50 51 Capar & Arı Aydınbelge52 reported that PUI presented lower SL scores than SI. Conversely, R�dig et al.53 reported significantly greater SL removal with the SI method compared to PUI. In turn, Jensen et al.19 reported no significant difference in cleaning efficiency between SI and PUI techniques. In this study, there was no significant difference between PUI and SI techniques. This inconsistency can be explained by the varying thickness and amount of SL depending on the different root canal file systems and irrigation protocols used during the preparation. LAI eliminates excessive enlargement of the root canals, and placing the laser tip in the coronal portion of the pulp chamber generates the spreading of photoacoustic waves.15
Koch et al.54stated that LAI with the PIPS technique increased fluid flow more than ultrasonic activation. Recent studies55, 56 reported that using PIPS resulted in excellent debris and SL removal without thermal damage to the dentin surface. Di Vito et al.57 reported that EDTA activation with PIPS had more cleaning efficiency than conventional irrigation. Likewise, in Ayranci et al.�s study,58 the activation of NaOCl and EDTA by PIPS resulted in more successful SL removal than PUI with the same irrigants. Conversely, Akyuz et al.14 found no significant difference between PIPS and SI. Similarly, the present study found no statistically significant differences between PIPS, PUI, and SI activation techniques. Therefore, the null hypothesis was accepted. This finding may result from the different percentages, types, and amounts of irrigants used and the duration of the activation procedure. Further research is required to establish a standardized protocol for the activation time, irrigants, and type of activation.
This study had some limitations, such as SEM evaluation of only 2D constructed areas of the root canal space, including sclerotic dentin. The area of sclerotic dentin reduces SL removal by irrigants, which can affect the study�s outcome. In SEM imaging, similar areas of canal walls were evaluated, but obtaining images in the same areas is challenging. Also, it is difficult to measure the thickness of the residual SL with SEM imaging. Moreover, SL production depends on the instrumentation.
However, untouched root canal walls usually remain the same during preparation. A significant limitation of our study was its laboratory-based environment. It is crucial to note that the results may vary slightly if the same procedures are used on a living being. Based on the findings presented here, it is imperative to devise alternative protocols to better prepare the dentinal canal wall for adhesive resin cementation in endodontically treated teeth for future research.
Conclusions
The results of this investigation showed that every IAT used was successful in statistically lowering the SL. Nevertheless, no irrigation activation method was able to remove the entire SL.
�
References
1. Vichi A, Grandini S, Ferrari M. Comparison between two clinical procedures for bonding fiber posts into a root canal: a microscopic investigation. J Endod. 2002;28:355-60.
2. Seefeld F, Wenz HJ, Ludwig K, Kern M. Resistance to fracture and structural characteristics of different fiber reinforced post systems. Dent Mater. 2007;23:265-71.
3. Lassila LV, Tanner J, Le Bell A-M, Narva K, Vallittu PK. Flexural properties of fiber reinforced root canal posts. Dent Mater. 2004;20:29-36
4. Gu XH, Mao CY, Kern M. Effect of different irrigation on smear layer removal after post space preparation. J Endod. 2009;35:583-6.
5. Hosaka K, Prasansuttiporn T, Thanatvarakorn O, Kunawarote S, Takahashi M, Foxton RM, et al. Smear layerdeproteinization: improving the adhesion of self-etch adhesive systems to caries-affected dentin. Curr Oral Health Rep. 2018;5:169-77.
6. Schmidt TF, Teixeira CS, Felippe MC, Felippe WT, Pashley DH, Bortoluzzi EA. Effect of ultrasonic activation of irrigants on smear layer removal. J Endod. 2015;41:1359-63.
7. Saber SE-D, Hashem AAR. Efficacy of different final irrigation activation techniques on smear layer removal. J Endod. 2011;37:1272-5.
8. Mohammadi Z, Shalavi S, Yaripour S, Kinoshita JI, Manabe A, Kobayashi M, et al. Smear layer removing ability of root canal irrigation solutions: a review. J Contemp Dent Pract. 2019;20:395-402.
9. Breschi L, Martin P, Mazzoni A, Nato F, Carrilho M, Tj�derhane L, et al. Use of a specific MMP-inhibitor (galardin) for preservation of hybrid layer. Dent Mater. 2010;26:571-8.
10. Seseogullari-Dirihan R, Mutluay MM, Vallittu P, Pashley DH, Tezvergil-Mutluay A. Effect of pretreatment with collagen crosslinkers on dentin protease activity. Dent Mater. 2015;31:941-7.
11. Mountouris G, Silikas N, Eliades G. Effect of sodium hypochlorite treatment on the molecular composition and morphology of human coronal dentin. J Adhes Dent. 2004;6:175-82.
12. O�Connell MS, Morgan LA, Beeler WJ, Baumgartner JC. A comparative study of smear layer removal using diferente salts of EDTA. J Endod. 2000;26:739-43.
13. Saikaew P, Sattabanasuk V, Harnirattisai C, Chowdhury AFMA, Carvalho R, Sano H. Role of the smear layer in adhesive dentistry and the clinical applications to improve bonding performance. Jpn Dent Sci Rev. 2022;58:59-66.
14. Akyuz Ekim SN, Erdemir A. Comparison of different irrigation activation techniques on smear layer removal: an in vitro study. Microsc Res Tech. 2015;78:230-9.
15. Ozbay Y, Erdemir A. Effect of several laser systems on removal of smear layer with a variety of irrigation solutions. Microsc Res Tech. 2018;81:1214-22.
16. Macedo R, Verhaagen B, Rivas DF, Versluis M, Wesselink P, van der Sluis L. Cavitation measurement during sonic and ultrasonic activated irrigation. J Endod. 2014;40:580-3.
17. Conde A, Estevez R, Loro�o G, Valencia de Pablo O, Rossi‐ Fedele G, Cisneros R. Effect of sonic and ultrasonic activation on organic tissue dissolution from simulated grooves in root canals using sodium hypochlorite and EDTA. Int Endod J. 2017;50:976-82.
18. Wiseman A, Cox TC, Paranjpe A, Flake NM, Cohenca N, Johnson JD. Efficacy of sonic and ultrasonic activation for removal of calcium hydroxide from mesial canals of mandibular molars: a microtomographic study. J Endod. 2011;37:235-8.
19. Jensen SA, Walker TL, Hutter JW, Nicoll BK. Comparison of the cleaning efficacy of passive sonic activation and passive ultrasonic activation after hand instrumentation in molar root canals. J Endod. 1999;25:735-8.
20. Aldeen RZ, Aljabban O, Milly H, Allouch A, Hamadah O. Effect of Er:YAG laser-activated irrigation on dentine debris removal from different parts of the root canal system: an in vitro study. Dent Med Probl. 2018;55:133-8.
21. Verstraeten J, Jacquet W, De Moor R, Meire M. Hard tissue debris removal from the mesial root canal system of mandibular molars with ultrasonically and laser-activated irrigation: a micro-computed tomography study. Lasers Med Sci. 2017;32:1965-70.
22. Korkut E, Torlak E, Gezgin O, �zer H, Şener Y. Antibacterial and smear layer removal efficacy of Er: YAG laser irradiation by photon-induced photoacoustic streaming in primary molar root canals: a preliminary study. Photomed Laser Surg. 2018;36:480-6.
23. Akcay M, Arslan H, Mese M, Durmus N, Capar ID. Effect of photon-initiated photoacoustic streaming, passive ultrasonic, and sonic irrigation techniques on dentinal tubule penetration of irrigation solution: a confocal microscopic study. Clin Oral Investig. 2017;21:2205-12.
24. Wen C, Yan L, Kong Y, Zhao J, Li Y, Jiang Q. The antibacterial efficacy of photon-initiated photoacoustic streaming in root canals with different diameters or tapers. BMC Oral Health. 2021;21:542.
25. Swimberghe RCD, Tzourmanas R, De Moor RJG, Braeckmans K, Coenye T, Meire MA. Explaining the working mechanism of laser‐activated irrigation and its action on microbial biofilms: a high‐speed imaging study. Int Endod J. 2022;55:1372-84.
26. Nejc L, Matija J. Amplification of pressure waves in laserassisted endodontics with synchronized delivery of Er: YAG laser pulses. Lasers Med Sci. 2018;33:823-33.
27. Mancini M, Cerroni L, Palopoli P, Olivi G, Olivi M, Buoni C, et al. FESEM evaluation of smear layer removal from conservatively shaped canals: laser activated irrigation (PIPS and SWEEPS) compared to sonic and passive ultrasonic activation-an ex vivo study. BMC Oral Health. 2021;21:1-10.
28. Obeid M, Elshaboury E, Obeid R. A comparative SEM assessment for the ability of PIPS, XP-Finisher and PUI to eliminate smear layer and open dentinal tubules. Egypt Dent J. 2022;68:1907-16.
29. Faul F, Erdfelder E, Lang AG, Buchner A. G* Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175-91.
30. Roitman ML, Picca M, Macchi RL. Post preparation: cleanness achieved by different irrigating protocols. Acta Odontol Latinoam. 2020;33:117-24.
31. Ko�ak S, Bağcı N, �i�ek E, T�rker SA, Sağlam BC, Ko�ak MM. Influence of passive ultrasonic irrigation on the efficiency of various irrigation solutions in removing smear layer: a scanning electron microscope study. Microsc Res Tech. 2017;80:537-42.
32. Gu L, Kim JR, Ling J, Choi KK, Pashley DH, Tay FR. Review of contemporary irrigant agitation techniques and devices. J Endod. 2009;35:791-804.
33. Van Meerbeek B, De Munck J, Yoshida Y, Inoue S, Vargas M, Vijay P, van Landuyt K, Lambrechts P, Vanherle G. Buonocore memorial lecture. Adhesion to enamel and dentin: current status and future challenges. Oper Dent. 2003;28:215-35.
34. Al-Shammari MG. Regenerative endodontic procedure in immature permanent teeth. In: Moolla A editor. Clinical Concepts and Practical Management Techniques in Dentistry. IntechOpen, 2022.
35. Lo Giudice G, Lizio A, Giudice RL, Centofanti A, Rizzo G, Runci M, Alibrandi A, Cicci� M. The effect of different cleaning protocols on post space: A SEM study. Int J Dent. 2016;2016:1907124.
36. Abarajithan M, Dham S, Velmurugan N, Valerian-Albuquerque D, Ballal S, Senthilkumar H. Comparison of Endovac irrigation system with conventional irrigation for removal of intracanal smear layer: an in vitro study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112:407-11.
37. Rahimi S, Janani M, Lotfi M, Shahi S, Aghbali A, Vahid Pakdel M, et al. A review of antibacterial agents in endodontic treatment. Iran Endod J. 2014;9:161-8.
38. Johnson GS, Mucalo MR, Lorier MA. The processing and characterization of animal-derived bone to yield materials with biomedical applications Part 1: Modifiable porous implants from bovine condyle cancellous bone and characterization of bone materials as a function of processing. J Mater Sci Mater Med. 2000;11:427-41.
39. Xiaoli H, Junqi L, Yan G. Effects of irrigation solutions on dentin wettability and roughness. J Endod. 2010;36:1064-7.
40. da Cunha LF, Furuse AY, Mondelli RFL, Mondelli J. Compromised bond strength after root dentin deproteinization reversed with ascorbic acid. J Endod. 2010;36:130-4.
41. Niu W, Yoshioka T, Kobayashi C, Suda H. A scanning electron microscopic study of dentinal erosion by final irrigation with EDTA and NaOCl solutions. Int Endod J. 2002;35:934-9.
42. De-Deus G, Namen F, Galan J, Zehnder M. Soft chelating irrigation protocol optimizes bonding quality of Resilon/ Epiphany root fillings. J Endod. 2008;34:703-5.
43. Dotto L, Onofre RS, Bacchi A, Pereira GKR. Effect of root canal irrigants on the mechanical properties of endodontically treated teeth: a scoping review. J Endod. 2020;46:596-604.
44. Chow TW. Mechanical effectiveness of root canal irrigation. J Endod. 1983;9:475-9.
45. Munoz HR, Camacho-Cuadra K. In vivo efficacy of three different endodontic irrigation systems for irrigant delivery to working length of mesial canals of mandibular molars. J Endod. 2012;38:445-8.
46. Shahravan A, Haghdoost A, Adl A, Rahimi H, Shadifar F. Effect of smear layer on sealing ability of canal obturation: a systematic review and meta-analysis. J Endod. 2007;33:96-105.
47. Lee SJ, Wu MK, Wesselink P. The effectiveness of syringe irrigation and ultrasonics to remove debris from simulated irregularities within prepared root canal walls. Int Endod J. 2004;37:672-8.
48. Mancini M, Cerroni L, Iorio L, Armellin E, Conte G, Cianconi L. Smear layer removal and canal cleanliness using diferente irrigation systems (EndoActivator, EndoVac, and passive ultrasonic irrigation): field emission scanning electron microscopic evaluation in an in vitro study. J Endod. 2013;39:1456-60.
49. Silva E, Rover G, Belladonna FG, Herrera DR, De-Deus G, Fidalgo T. Effectiveness of passive ultrasonic irrigation on periapical healing and root canal disinfection: a systematic review. Br Dent J. 2019;227:228-34.
50. Alves FR, Almeida BM, Neves MA, Moreno JO, R��as IN, Siqueira Jr JF. Disinfecting oval-shaped root canals: effectiveness of different supplementary approaches. J Endod. 2011;37:496-501.
51. Paix�o S, Rodrigues C, Grenho L, Fernandes MH. Efficacy of sonic and ultrasonic activation during endodontic treatment: a meta-analysis of in vitro studies. Acta Odontol Scand. 2022;80:588-95.
52. Capar ID, Arı Aydınbelge H. Effectiveness of various irrigation activation protocols and the self-adjusting file system on smear layer and debris removal. Scanning. 2014;36:640-7.
53. R�dig T, D�llmann S, Konietschke F, Drebenstedt S, H�lsmann M. Effectiveness of different irrigant agitation techniques on debris and smear layer removal in curved root canals: a scanning electron microscopy study. J Endod. 2010;36:1983-7.
54. Koch JD, Jaramillo DE, DiVito E, Peters OA. Irrigant flow during photon-induced photoacoustic streaming (PIPS) using Particle Image Velocimetry (PIV). Clin Oral Investig. 2016;20:381-6.
55. Sippus J, Gutknecht N. Deep disinfection and tubular smear layer removal with Er:YAG using photon-induced photoacoustic streaming (PIPS) contra laser-activated irrigation (LAI) technics. Lasers Dent Sci. 2019;3:37-42.
56. Al Shahrani M, DiVito E, Hughes CV, Nathanson D, Huang GT. Enhanced removal of Enterococcus faecalis biofilms in the root canal using sodium hypochlorite plus photon-induced photoacoustic streaming: an in vitro study. Photomed Laser Surg. 2014;32:260-6.
57. DiVito E, Peters OA, Olivi G. Effectiveness of the Erbium:YAG laser and new design radial and stripped tips in removing the smear layer after root canal instrumentation. Lasers Med Sci. 2012;2(27):273-80.
58. Ayranci L, Arslan H, Akcay M, Capar I, Gok T, Saygili G. Effectiveness of laser‐assisted irrigation and passive ultrasonic irrigation techniques on smear layer removal in middle and apical thirds. Scanning. 2016;38:121-7.
�
K�rşat Er
E-mail address: qursater@hotmail.com
�
Conflict of interest
The authors have no conflicts of interest to declare.
�
Ethical disclosures
Protection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of data. The authors declare that no patient data appear in this article.
Right to privacy and informed consent. The authors declare that no patient data appear in this article.
�
CRediT authorship contribution statement
Dide Tekinarslan: Conceptualization, Data Curation, Investigation, Methodology, Visualization, Validation, Writing � Original Draft, Writing � Review and Editing. Damla Erkal: Investigation, Methodology, Validation, Writing � Review and Editing. Simay Ko�: Investigation, Methodology, Validation, Writing � Review and Editing. Hakan Er: Methodology, Validation, Writing � Original Draft. K�rşat Er: Conceptualization, Methodology, Supervision, Validation, Visualization, Writing � Original Draft, Writing � Review and Editing.
�
Acknowledgment
We would like to express our sincere gratitude to Vural Aslan, Ali Umut Akkuş, and Mustafa R�zgar for their invaluable assistance in the collection and storage of samples. Their dedication and support were instrumental in the successful completion of this study.
�
1646-2890/� 2024 Sociedade Portuguesa de Estomatologia e Medicina Dent�ria. Published by SPEMD.
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
