
Revista Portuguesa de Estomatologia, Medicina Dentária e Cirurgia Maxilofacial
SPEMD - Revista Portuguesa de Estomatologia Medicina Dentária e Cirurgia Maxilofacial | 2024 | 65 (2) | 94-98
Clinical case
White Sponge Nevus of the oral mucosa – Case report
Nevo Branco Esponjoso da mucosa oral – Relato de caso
a Faculdade de Medicina Dentária, Universidade de Lisboa, Lisboa, Portugal
Beatriz Batalha - abbm@campus.ul.pt
Article Info
Rev Port Estomatol Med Dent Cir Maxilofac
Volume - 65
Issue - 2
Clinical case
Pages - 94-98
Go to Volume
Article History
Received on 13/07/2023
Accepted on 18/05/2024
Available Online on 18/06/2024
Keywords
Clinical Case Report
�
White Sponge Nevus of the oral mucosa � Case report
Nevo Branco Esponjoso da mucosa oral � Relato de caso
�
Beatriz Batalha1,* 0000-0002-8471-1031
Daniela Abreu1 0000-0002-7102-905X
Andr� Moreira1 0000-0001-6699-1626
Filipe Freitas1 0000-0002-0024-9186
Helena Francisco1 0000-0002-6783-7740
Jo�o Caram�s1 0000-0002-5544-3744
1 Faculdade de Medicina Dent�ria, Universidade de Lisboa, Lisboa, Portugal
�
�
Article history:
Received 13 July 2023
Accepted 18 May 2024
Available online 11 June 2024
�
Abstract
White sponge nevus (WSN) is a rare autosomal-dominant disorder of the non-keratinized mucosa, characterized by a thickened white edematous mucosa whose surface appears irregular, wrinkly, or spongy. It affects mainly the oral mucosa. The histopathological examination demonstrates hyperparakeratosis, acanthosis with intracellular edema and vacuolization over the prickle cell layer, and eosinophilic perinuclear condensation. The currently accepted etiology is a mutation of the keratin 4 and/or keratin 13 genes. We present a case of WSN diagnosed in a 30-year-old white male patient with white corrugated bilateral lesions on the buccal mucosa that extended beyond the occlusal plane. A biopsy was obtained, and the histological presentation included superficial parakeratosis, prominent acanthosis of the squamous epithelium, and areas with prominent intracellular edema, which confirmed the diagnosis. No treatment was necessary other than patient counseling and initial 6-month follow-up consultation. His attending dentist was informed of the diagnosis, and further follow-ups were recommended.
Keywords: Autosomal dominant disorder,Oral mucosa,White lesions,White sponge nevus
�
Resumo
O nevo branco esponjoso (NBE) � uma doen�a rara, autoss�mica dominante, da mucosa n�o queratinizada, que se caracteriza pela presen�a de uma mucosa espessada e esbranqui�ada cuja superf�cie � irregular, rugosa ou esponjosa. A mucosa oral � a mais afetada.
Na avalia��o histopatol�gica apresenta hiper-paraqueratose, acantose com edema e vacuoliza��o intracelulares na camada espinhosa, e condensa��o eosinof�lica perinuclear. Atualmente pensa-se que a etiologia resulta da muta��o dos genes da queratina 4 e/ou 13. Apresentamos um caso de um homem, leucod�rmico, de 30 anos, com les�es brancas enrugadas bilaterais na mucosa bucal, cuja extens�o ultrapassa o plano oclusal. Foi realizada uma biopsia, e a apar�ncia histol�gica traduziu-se em paraqueratose superficial, com proeminente acantose do epit�lio pavimentoso e �reas de proeminente edema intracelular, o que confirmou o diagn�stico. N�o foi necess�rio tratamento, para al�m do aconselhamento do doente e follow-up inicial de 6 meses. O seu m�dico dentista foi informado do diagn�stico e recomendou-se manuten��o de controlos
Palavras-chave: Doen�a autoss�mica dominante,Mucosa oral,Les�es brancas, Nevo branco esponjoso
�
Introduction
White sponge nevus (WSN) is a rare but benign hereditary autosomal-dominant genodermatosis of the non-keratinized mucosa.1 - 11 It affects 1 in 200.000 people,10 - 12 and most cases affect the buccal mucosa bilaterally, although the lips, alveolar ridges, and floor of the mouth may also be involved.3 5 -
7 10 12 - 14Extraoral presentation sites are the nasal, esophageal, rectal, and genital mucosa.1, 3, 5, 7 - 10, 13, 15 - 17 WSN clinical landmarks are thickened white edematous mucosa whose surface is irregular, wrinkly, or spongy in appearance.3 - 10 13 WSN�s histopathological examination shows hyperparakeratosis, acanthosis with intracelular edema and vacuolization over the prickle cell layer, and eosinophilic perinuclear condensation from keratin tonofilament deposition.5, 10, 13 The exact etiology remains uncertain, but mutations in keratin-4 (KRT4) or keratin-13 (KRT13) genes are believed to be the cause, as these genes are responsible for the production of cytokeratins specifically expressed in the spinous cells layer of the mucosal epithelium. 5 - 10 15 16 18 19
Familial cases of WSN have been reported, but most of the published literature refers to single case reports, as there is incomplete penetrance of the trait.2, 8, 16 WSN diagnosis is clinical, but cytologic or histopathological confirmation should be sought. Given that it is a benign condition, no treatment is usually necessary.1, 3, 4, 6, 20
Case Report
A 30-year-old white male patient was referred to our clinic by his attending dentist. He presented with a 2-year history of non-removable bilateral white buccal mucosal lesions, which were asymptomatic. His dentist believed the lesions were associated with bruxism and, therefore, had given him a nightguard the previous year. As the lesions did not improve and seemed to be worsening, a course of local nystatin applied for 15 days was recommended to rule out possible oral candidiasis; however, without any clinical results.
Although his medical history was noncontributory to the presenting lesions, the patient was under treatment with Relvar� (fluticasone furoate/vilanterol) once per day and Avamys� (fluticasone furoate) as needed for his asthma. He also had a history of spine surgery and septoplasty and mentioned being allergic to Broncovaxon� (lyophilized bacterial lysates of Haemophilus influenzae, Diplococcus pneumoniae, Klebsiella pneumoniae, and ozaenae, Staphylococcus aureus, Streptococcus pyogens and viridans, and Neisseria catarrhalis) and Valtrex� (valaciclovir).
Intraoral examination revealed white corrugated bilateral lesions on the buccal mucosa that extended beyond the occlusal plane (Figure 1). Although there was mild sloughing on the most prominent lesions, they were not removable by scraping.
There were no similar lesions elsewhere in the remaining oral mucosa. Given the patient�s age and no known history of trauma, the clinical presentation, and the lesions� bilateral nature, we presumed a WSN diagnosis.
An incisional biopsy with an elliptical incision design (15c scalpel blade) under local anesthesia with 4% articaine and 1:200000 epinephrine (Artinibsa, INIBSA, Portugal) was performed. Four single stitches with resorbable sutures (Novosyn, PGA 4.0, B.BRAUN, Spain) were placed for primary wound closure.
The post-operative observation was conducted 15 days after (Figures 2 and 3). The specimen was preserved in a 10% buffered formalin solution and sent for histopathological examination, which identified superficial parakeratosis, promirev nent acanthosis of the squamous epithelium, and areas with prominent intracellular edema. These findings are consistente with our diagnosis of oral WSN (Figures 4 and 5). No genetic study was performed. WSN is a benign condition that requires no treatment.
�
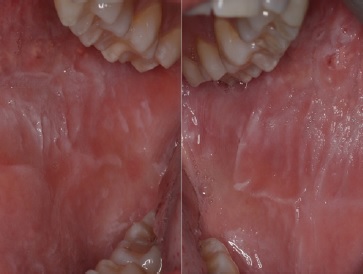
Figure 1. Intraoral presentation of the lesions, showing the white diffuse corrugated, spongy plaques and their extension on the right and left buccal mucosae.
�
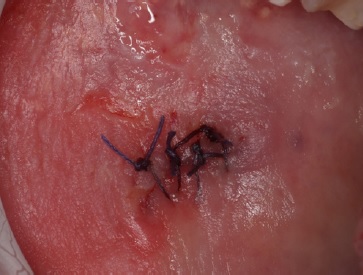
Figure 2. The oral biopsy site on the right buccal mucosa encompassing both altered and apparently sound mucosa and wound closure.
�
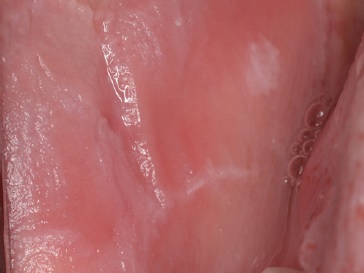
Figure 3. Post-operative presentation at 15 days. Sutures were no longer present.
�
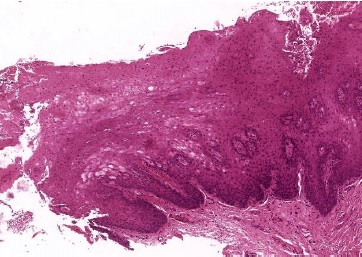
Figure 4. Microscopic features of the specimen retrieved from the right buccal mucosa biopsy (hematoxylineosin staining, 40x) showing superficial parakeratosis and prominent acanthosis of the squamous epithelium.
�
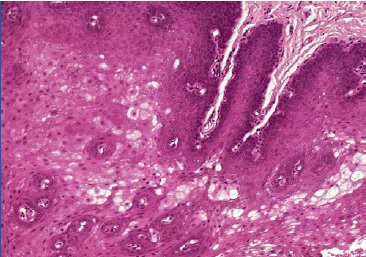
Figure 5. Microscopic features of the specimen retrieved from the right buccal mucosa biopsy (hematoxylin-eosin staining, 100x) showing prominent intracellular edema.
�
As it may manifest in other areas, the patient was counseled and advised to inform his attending physicians. No follow-up appointments with our clinic were required after the initial 6-month follow-up. The attending dentist was informed, and further follow-up appointments were recommended.
After confirmation of the diagnosis, we questioned the patient again about his family, and he denied having any family history of such lesions. We could only clinically confirm the absence of such lesions on his brother, as the remaining family members lived abroad.
Discussion and Conclusions
WSN was first described by Hyde in 1909, but its current name was introduced by Cannon in 1935.1, 4, 5, 7, 16, 21 It is usually presente at birth, although it may develop during adolescence. We present a less common case of late adult diagnosis.
WSN is a rare autosomal-dominant disorder caused by germline variants of the keratin genes KRT4 or KRT13, located at chromosomes 12q13 and 17q21-q22, respectively.18, 22, 23 The keratin filaments resulting from these germline mutations can be easily damaged from light mechanical trauma, causing cytokine flooding of the basal cell layer, which induces excessive basal cell proliferation and, subsequently, mucosal hyperkeratosis.16 KRT13 variants seem to be exclusively responsible for the extraoral site involvement. 8, 22
WSN does not present gender or ethnic predilection.1, 2, 4, 19 It is a non-painful condition characterized by white irregular, thick, spongy plaques on mucosal tissues, mainly in the oral mucosa.1, 3 - 5, 7, 12 - 14, 20 It can also arise in the nasal, esophageal, rectal, and genital mucosa, especially the vaginal mucosa.1, 3 - 5, 7 - 9, 13, 15 - 17 However, most cases involve the oral mucosa alone.1, 28
Different authors have proposed various names to describe this condition over the years. Published denominations still found in the literature are �congenital leukokeratosis mucosa oris� proposed by Luda and Shirazy in 1941, �leukokeratosis oris� proposed by Lynch in 1951, �nevus spongiosus albus mucosae� proposed by Gandy in 1952, �white folded gingiva- stomatosis� proposed by Everett and Noyes in 1953, �pachyderma oralis� proposed by Kinney and Derifield in 1956, �familial white folded hypertrophy of the mucous membranes� proposed by Zegarelli and Kutscher in 1957, �familial white folded gingivostomatosis� proposed by Darling and Fletcher in 1958, �white folded dysplasia of the mucous membranes� proposed by Zagareli et al. in 1959, and �hereditary leukokeratosis� proposed by Scott in 1967.1, 4
When lesions are identified at birth, the differential diagnoses should include pachyonychia congenita, hereditary benign intraepithelial dyskeratosis, Darier�s disease, and dyskeratosis congenita. When lesions are exclusive to the oral cavity and the diagnosis occurs later in life, the main differential diagnoses to consider are leukoedema, oral candidiasis, morsicatio buccarum, oral lichen planus, leukoplakia, proliferative verrucous leukoplakia, and oral squamous cell carcinoma.4, 5, 9, 10, 20
The literature is controversial regarding WSN�s malignant transformation potential, although it is considered a benign condition. Downham et al.24 reported a case of malignant transformation associated with long-term prednisone use. In turn, de Haseth et al.22 reported a large four-generation family cohort of 12 affected individuals, of which three presented abnormal cervical smears at relatively young ages (16, 18, and 27 years) and without apparent risk factors, and two had developed oral squamous cell carcinoma. After further analysis, a significant downregulation of KRT4 and KRT13 expression was demonstrated in this family�s oral squamous cell carcinomas, suggesting a possible relationship between missense mutations in genes KRT4 and/or KRT13 and the development of malignant oral lesions. Whether this finding was incidental or there is a potential malignant transformation risk in WSN remains to be elucidated. Sarode et al.23 revisited this question regarding their findings in the histopathological evaluation of an excised oral squamous cell carcinoma in an undiagnosed WSN, highlighting that the role of the downregulation of KRT4 and KRT13 in both WSN and oral squamous cell carcinoma needs further investigation.
The WSN histopathological findings are a thickened stratified squamous epithelium showcasing parakeratosis, cytoplasmatic clarification, and vacuolization due to the intracelular edema of the stratum spinosum layer, nuclear condensation, very mild inflammatory infiltrate, if present, and eosinophilic perinuclear aggregates.3, 5, 10, 13 These appear to be unique to WSN and represent the aggregation of keratin tonofilaments, as shown in ultrastructural studies by electron microscopy.1, 3, 10, 13 The value of cytology alone in the diagnosis of WSN is controversial. Cohen et al.1 found their results on exfoliative cytology to be unspecific and, therefore, of limited diagnostic value. In turn, Martins Filho et al.12 confirmed the presence of perinuclear eosinophilic condensation on their smears, adding weight to Messadi et al.�s25 claims that the characteristic perinuclear eosinophilic condensations are more visible on smears than on conventional histopathological examinations. Lajolo et al.26 have suggested using exfoliative cytology with KRT4 and KRT13 gene sequencing instead of oral biopsy as the diagnostic algorithm of choice, as it is a non-invasive approach, thus preferable for younger patients.
WSN treatment is not necessary.1, 3, 4, 6, 9, 11, 12, 20, 25 Nonetheless, studies have reported multiple treatment attempts (vitamins, antihistamines, nystatin, parental penicillin, ampicillin, topical tretinoin) with very low or unsuccessful outcomes.5, 27 Interestingly, some reports have shown improvement with tetracycline mouth rinses when clinical complaints are present.5, 14, 27, 28 WSN�s epithelial folds are presumed to potentially promote an ideal environment for microbial growth of otherwise non-pathogenic organisms, which could explain the apparent effectiveness of topical antibiotics.27 Another hypothesis relates to the possible modulation of epithelial keratinization by tetracyclines since they demonstrate inhibitory effects not only on bacterial protein synthesis but also on neutrophil chemotaxis, lymphocyte mitogenic response, and prostaglandin synthesis.14, 28
WSN is a rare condition that should not be forgotten as one of the differential diagnoses of oral white lesions. Defective keratinization of the oral mucosa due to mutations in the KRT4 or KRT13 genes is the current accepted etiology. WSN characteristic clinical appearance involving mainly the buccal mucosa with its thickened white edematous, irregular, wrinkly, or spongy surface lesions should be recognized by all oral healthcare professionals to avoid misdiagnosis and unnecessary treatments and contribute to an early diagnosis. Given the multitude of differential diagnoses for white lesions of the oral mucosa, when in doubt, the general dental practitioner should promptly refer these patients to an Oral Medicine specialist for adequate diagnosis. Definitive diagnosis should be confirmed by biopsy or cytology with KRT4 and KRT13 gene sequencing.
WSN is defined as a benign condition, so no treatment is required other than informing and reassuring the patient. However, given the controversies on whether there may be a potential for malignant transformation, patient education on oral cancer risk factors is advisable, and follow-up should be encouraged in high-risk patients.
�
References
1. Cohen L, Young AH. The white sponge naevus. Br J Oral Surg. 1968;5:206-10.
2. Kamalamma MK, Prabhu SR, Shetty JN, Rao NR. The white sponge nevus. Oral Surg Oral Med Oral Pathol. 1970;30:51-4.
3. Frithiof L, B�n�czy J. White sponge nevus (leukoedema exfoliativum mucosae oris): ultrastructural observations. Oral Surg Oral Med Oral Pathol. 1976;41:607-22.
4. Miller CS, Craig RM Jr. White corrugated mucosa. J Am Dent Assoc. 1988;117:345-6.
5. Martelli H Jr, Pereira SM, Rocha TM, Nogueira dos Santos PL, Batista de Paula AM, Bonan PR. White sponge nevus: report of a three-generation family. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:43-7.
6. L�pez Jornet P. White sponge nevus: presentation of a new family. Pediatr Dermatol. 2008;25:116-7.
7. Aghbali A, Pouralibaba F, Eslami H, Pakdel F, Jamali Z. White sponge nevus: a case report. J Dent Res Dent Clin Dent Prospects. 2009;3:70-2.
8. Kimura M, Nagao T, Machida J, Warnakulasuriya S. Mutation of keratin 4 gene causing white sponge nevus in a Japanese family. Int J Oral Maxillofac Surg. 2013;42:615-8.
9. Benoit S, Schlipf N, Hausser I, Fischer J, Hamm H. White sponge nevus � a rare autosomal dominant keratinopathy. Klin Padiatr. 2014;226:375-6.
10. Sanjeeta N, Nandini DB, Premlata T, Banerjee S. White sponge nevus: Report of three cases in a single family. J Oral Maxillofac Pathol. 2016;20:300-3.
11. Bezerra KT, Leite TC, Roza ALOC, Ara�jo R, Israel MS, Canedo NHS, et al. White sponge nevus: A condition not always clinically suspected. J Cutan Pathol. 2020;47:22-6.
12. Martins Filho PR, Brasileiro BF, Piva MR, Trento CL, Santos TS. Familial case of oral white sponge nevus--a rare hereditary condition. An Bras Dermatol. 2011;86(4 Suppl 1):S39-S41.
13. McGininis JP Jr, Turner JE. Ultrastructure of the white sponge nevus. Oral Surg Oral Med Oral Pathol. 1975;40:644-51.
14. Otobe IF, Sousa SO, Migliari DA, Matthews RW. Successful treatment with topical tetracycline of oral white sponge nevus occurring in a patient with systemic lupus erythematosus. Int J Dermatol. 2006;45:1130-1.
15. Rugg EL, McLean WH, Allison WE, et al. A mutation in the mucosal keratin K4 is associated with oral white sponge nevus. Nat Genet. 1995;11:450-2.
16. Shibuya Y, Zhang J, Yokoo S, Umeda M, Komori T. Constitutional mutation of keratin 13 gene in familial white sponge nevus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96:561-5.
17. Pangti R, Gupta S. White Sponge Nevus of Glans Penis. J Cutan Med Surg. 2022;26:215.
18. Terrinoni A, Rugg EL, Lane EB, Melino G, Felix DH, Munro CS, et al. A novel mutation in the keratin 13 gene causing oral white sponge nevus. J Dent Res. 2001;80:919-23.
19. Cai W, Jiang B, Yu F, Yang J, Chen Z, Liu J, et al. Current approaches to the diagnosis and treatment of white sponge nevus. Expert Rev Mol Med. 2015;17:e9.
20. Lucchese A, Favia G. White sponge naevus with minimal clinical and histological changes: report of three cases. J Oral Pathol Med. 2006;35:317-9.
21. Warin RP. Case for diagnosis; white sponge naevus of the mouth. Proc R Soc Med. 1953;46:285.
22. Haseth SB, Bakker E, Vermeer MH, Idriss HE, Bosse T, Smit VTHBM, et al. A novel keratin 13 variant in a four-generation family with white sponge nevus. Clin Case Rep. 2017;5:1503-9.
23. Sarode G, Sarode SC, Sharma NK. Phenotypic reflection of white sponge nevus in histomorphological features of oral squamous cell carcinoma. Oral Oncol. 2022;125:105707.
24. Downham TF Jr, Plezia RA. Oral squamous-cell carcinoma within a white-sponge nevus. J Dermatol Surg Oncol. 1978;4:470-2.
25. Messadi DV, Waibel JS, Mirowski GW. White lesions of the oral cavity. Dermatol Clin. 2003;21:63-78.
26. Lajolo C, Cafiero C, Stigliano E, Grippaudo FR, Chiurazzi P, Grippaudo C. Exfoliative Cytology and Genetic Analysis for a Non-Invasive Approach to the Diagnosis of White Sponge Nevus: Case Series. Bioengineering (Basel). 2023;10:154.
27. Lim J, Ng SK. Oral tetracycline rinse improves symptoms of white sponge nevus. J Am Acad Dermatol. 1992;26:1003-5.
28. Otobe IF, de Sousa SO, Matthews RW, Migliari DA. White sponge naevus: improvement with tetracycline mouth rinse: report of four cases. Clin Exp Dermatol. 2007;32:749-51.
�
Beatriz Batalha
E-mail address: joao.vitor.pereira@uel.br
�
Conflict of interest
The authors have no conflicts of interest to declare.
�
Ethical disclosures
Protection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of data. The authors declare that they have followed their work center protocols on access to patient data and for its publication.
Right to privacy and informed consent. The authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
�
CRediT authorship contribution statement
Beatriz Batalha: Conceptualization, Investigation, Visualization, Writing � original draft. Daniela Abreu: Conceptualization, Visualization, Writing � original draft. Andr� Moreira: Writing � review & editing. Filipe Freitas: Conceptualization, Investigation, Supervision, Writing � review & editing. Helena Francisco: Writing � review & editing. Jo�o Caram�s: Writing � review & editing.
�
1646-2890/� 2024 Sociedade Portuguesa de Estomatologia e Medicina Dent�ria. Published by SPEMD.
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
