
Revista Portuguesa de Estomatologia, Medicina Dentária e Cirurgia Maxilofacial
SPEMD - Revista Portuguesa de Estomatologia Medicina Dentária e Cirurgia Maxilofacial | 2023 | 64 (2) | 72-77
Original research
The Road to Sustainability in Dentistry – Is the Reuse of Sterilization Pouches Viable?
O Caminho para a Sustentabilidade em Medicina Dentária – Será a reutilização de Mangas de Esterilização Viável?
a Faculty of Dental Medicine, University of Porto, Porto, Portugal
b CEB, Center of Biological Engineering, LIBRO-Laboratório de Investigação em Biofilmes Rosário Oliveira, University of Minho, Braga, Portugal
c LABBELS–Associate Laboratory, Braga, Portugal
d Department of Dental Anatomy, Faculty of Dental Medicine, University of Porto, Porto, Portugal
e Department of Orthodontics, Faculty of Dental Medicine, University of Porto, Porto, Portugal
Catarina Amaral - caprf@hotmail.com
Article Info
Rev Port Estomatol Med Dent Cir Maxilofac
Volume - 64
Issue - 2
Original research
Pages - 72-77
Go to Volume
Article History
Received on 10/11/2022
Accepted on 16/06/2023
Available Online on 03/07/2023
Keywords
Original Research
�
The Road to Sustainability in Dentistry � Is the Reuse of Sterilization Pouches Viable?
O Caminho para a Sustentabilidade em Medicina Dent�ria � Ser� a reutiliza��o de Mangas de Esteriliza��o Vi�vel?
�
Catarina Amaral1,* 0000-0001-5471-8817
Mariana Henriques2,3 0000-0003-0317-4877
Fernanda Gomes2,3 0000-0003-0318-4733
Pedro Mesquita4 0000-0003-0735-2482
Maria Jo�o Ponces5 0000-0002-4341-4233
1 Faculty of Dental Medicine, University of Porto, Porto, Portugal
2 CEB, Center of Biological Engineering, LIBRO-Laborat�rio de Investiga��o em Biofilmes Ros�rio Oliveira, University of Minho, Braga, Portugal
3 LABBELS�Associate Laboratory, Braga, Portugal
4 Department of Dental Anatomy, Faculty of Dental Medicine, University of Porto, Porto, Portugal
5 Department of Orthodontics, Faculty of Dental Medicine, University of Porto, Porto, Portugal
�
�
Article history:
Received 10 November 2022
Accepted 16 June 2023
Available online 30 June 2023
�
Abstract
Objectives: To answer the question: Can sterilized pouches be used a second time while maintaining their sterility conditions?
Methods: This investigation tested paper/plastic sterilization pouches divided into three groups: experimental group � twice-used pouches; negative control group � once-used pouches; and positive control group � environmentally contaminated pouches. In the experimental group, pouches were opened, a gauze dressing was placed into them, and they were sterilized again, representing the reuse of the pouches (second sterilization cycle). After the sterilization cycle, samples were stored for 1 day (T0), 7 days (T1), 31 days (T2), and 153 days (T3). Positive control group pouches were opened and exposed to contamination in the storage environment. After the specified storage period, the experimental and negative control groups� pouches were opened, and the gauze dressings were removed aseptically. All gauze dressings of all groups, including the positive control group, were incubated in Petri dishes with nutrient agar at 37�C for 3 days. After incubation, the Petri dishes were inspected, and the microbial contamination was assessed and classified as present or absent.
Results: The experimental group�s Petri dishes showed no sign of contamination. The same happened to the negative control group. The positive control group�s Petri dishes presented microbial contamination. The same results were obtained for all incubation times.
Conclusions: This study showed that sterilization pouches could be used a second time while maintaining sterility and integrity conditions, even for extended periods (153 days � 5 months of storage).
Keywords: Dentistry, Environment, Equipment reuse, Paper/plastic pouches, Sterilization, Sustainable development, Waste Management
�
Resumo
Objetivos: Este estudo tinha como objetivo testar se as mangas de esteriliza��o poderiam ser utilizadas uma segunda vez mantendo as suas condi��es de esteriliza��o.
Métodos: Amostras de mangas de esterilização de papel/plástico foram testadas neste trabalho sendo divididas em 3 grupos (grupo experimental � mangas reutilizadas duas vezes; grupo de controlo negativo � mangas usadas uma vez; e um grupo de controlo positivo � amostras ambientalmente contaminadas). O grupo experimental incluiu mangas que foram abertas e uma foi gaze introduzida, sendo novamente fechadas e esterilizadas, representando assim a reutilização das mangas. Após o ciclo de esterilização, todas as amostras foram armazenadas durante 1 dia (T0), 7 dias (T1), 31 dias (T2) e 153 dias (T3). Quanto �s amostras do grupo de controlo positivo, ap�s o ciclo de esteriliza��o, estas foram abertas e expostas � contamina��o presente no ambiente de armazenamento. Após cada ciclo de armazenamento, as gazes foram incubadas em placas de Petri com Agar Nutriente a 37�C durante 3 dias. Após o período de incubação, as placas de Petri foram inspecionadas e a contaminação microbiana foi verificada e classificada como presente ou ausente.
Resultados: A observação das placas de Petri do grupo experimental não mostrou sinais de contaminação. O mesmo aconteceu com o grupo de controlo negativo. As restantes placas de Petri contendo os controlos positivos apresentaram contamina��o microbiana. Os mesmos resultados foram obtidos para todos os per�odos de incuba��o.
Conclus�es: Este estudo mostra que as mangas de esterilização podem ser utilizadas uma segunda vez, mantendo as suas condições de esterilidade e integridade mesmo para longos períodos de tempo (153 dias � 5 meses de armazenamento).
Palavras-chave: Odontologia, Ambiente, Reutiliza��o de equipamentos, Mangas de papel/pl�stico, Esteriliza��o, Desenvolvimento sustent�vel, Gest�o de res�duos
�
Introduction
Plastic in the medical sector was valued worldwide at 18.9 billion euros in 2019.1, 2 In Europe, this market is expected to reach a value of 4 billion euros by 2024 due to its growing demand.3 The World Health Organization (WHO) explains that 85% of the health sector waste is non-infectious. However, only a small percentage is recycled, while most end up in landfills (79%) or incinerated (12%). Consequently, the medical sector represents around 4% of global greenhouse gas emissions � if it were a country, it would be the fifth most polluting country in the world.4, 5
According to the Eco Dentistry Association (EDA), some 680 million plastic and paper protections and 1.7 billion sterilization instruments and packaging are sent to landfills or disposed of in the environment per year.6 - 8 This situation raises another problem: overexposure to bisphenol A (BPA) and di(2-ethylhexyl)phthalate (DEHP) and microplastics (small particles between 100 nm and 5 mm). Recent studies show that large amounts of microplastics end up in the human diet and have been found, for example, in seafood, honey, bottled water, and alcohol, as a result of their deterioration in the environment.3 - 5, 9 In our body, due to the inability of our immune system to eliminate plastic, this situation can lead to chronic inflammation and cancer.10 Also, the contact between microplastics and antibiotics in our body has adverse effects. In fact, the correlation between antimicrobial resistance and increasing plastic pollution is under investigation. The findings indicate that when in contact with antibiotics, plastics can promote genetic mutations in bacteria that cause them to acquire antibiotic resistance, creating possible threats to human health.11, 12
Reducing plastic consumption at hospitals and medical/ dental offices is a difficult task since medical areas have benefited most from its use.13 - 16 Plastic is economical, heat resistant, long-lasting, versatile, biocompatible, requires less energy to be produced compared to metal or glass, and offers a sterile environment.1, 13 These assets that make plastic the ideal material for single use also make it almost impossible for nature to eliminate.10,17 In addition, single-use plastics currently represent 85% of all plastics in health.13 Because recycling these plastics in the health sector carries risks of cross-infection and contamination, the current destruction methods aim to provide the population with more security.1, 8, 15 - 18
The recently published study �Environmental sustainability practices in Portuguese dental clinics� concluded that clinical directors showed good environmental awareness and satisfactory implementation of environmental sustainability practices in dental clinics and that costs were the most reportreved barrier to implementing further practices. This study triggered the interest in finding a safe, easy, cheap solution for dental clinics to implement that would also be sustainable and eco-friendly, allowing the mitigation of the effects of the waste produced by single-use plastic.17, 18
For the reasons stated above, sterilization pouches were considered the ideal specimen for this study because they do not come into contact with the patient and consist of a paper/plastic bag with one side made of medical-grade paper and the other made of a thin plastic film. They cover medical instruments before their sterilization in the autoclave and are currently thrown away after the sterilized instruments are unpacked.
The problem addressed is not only the usage of plastic but also how it is discarded. One of the complications related to reusing these plastics is that they are marked as single-use by manufacturers.19
In the impossibility of replacing plastic with other materials because of its many assets, this study aims to question the safety and efficacy of reusing sterilization pouches without compromising their sterilization conditions.20, 21 Therefore, the question and hypothesis raised are: Can sterilization pouches be used a second time while maintaining their sterilization conditions?
Material and methods
Paper/plastic pouches were prepared from non-sterile sterilization rolls (MEDISTOCK�, France), with 7.5x15.0 cm each, and randomly selected to undergo a sterilization cycle in na autoclave at 121�C and 15 psi for 33 minutes (HS�22 K5+ WHITE, GENTINGE�, Sweden) to mimic their use in dental practices and hospitals. After that cycle, pouches that met the inclusion criteria were selected as the Experimental Group (EG) and opened to undergo a second sterilization cycle (n=12). The inclusion criteria were not presenting any openings, water drops inside, bends and creases, or burns.
The pouches that did not pass the quality check to be reused �exclusion criterium� were discarded. A 3 cm x 2 cm non-sterilized gauze dressing (Bastos Viegas�, S. A., Portugal) was placed into each of the once-used pouches: EG pouches; Negative Control Group (NCG) � once-used pouches; and Positive Control Group (PCG) � environmentally contaminated pouches. Afterward, the NCG and the PCG pouches were sealed at 1 cm from the base and 3 cm from the top with a thermal sealer (EuroSeal� 2001, Euronda S.p.A., Italy), and the EG pouches were resealed at 6 cm from the top (because they were being reused) with the same sealer. In this step, the exclusion criteria were again applied to ensure/maintain integrity.
All pouches were arranged in a horizontal position, with the paper side of one pouch in contact with the plastic side of the next one without touching the chamber wall of the autoclave. The specimens were sterilized at 121�C and 15 psi for 33 minutes.
After the sterilization cycle, specimens were stored in na opened plastic box in a microbiology laboratory (Laboratory of Microbiology Applied to Health [LMAS], University of Minho) to recreate an open microorganism-rich environment, for 1 day (T0), 7 days (T1), 31 days (T2), and 153 days (T3) at room temperature (≅ 20�C) and humidity (≅ 40%). After each storage period, the pouches were inspected for barrier damage before being opened. Figure 1 shows the design of the study in a flow chart.
�
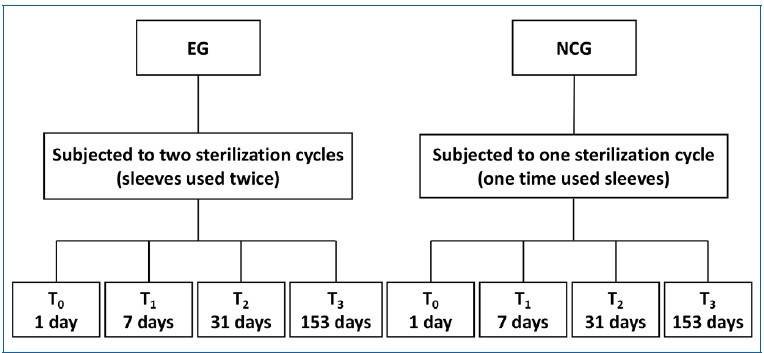
�
The gauze dressings were removed aseptically (MSC-Advantage� Class II Biological Safety Cabinets, Thermo Fisher Scientific�, USA) and incubated (general incubator with builtin roller or shaker � NB-205Q, N-BIOTEK, South Korea) in nutriente agar (Research Products International � RPI, USA) in Petri dishes at 37�C for 3 days. After incubation, the Petri dishes were inspected, and microbial contamination was assessed and classified as present or absent based on the visible changes in color and shape of the mediums.22 The results were compared regarding the different periods of storage and sample groups.
Lastly, the sample size determination was carried out in Microsoft Excel Spreadsheet Software, taking into consideration the following study objectives: evaluate the presence of contamination in each of the three groups (EG, NCG, and PCG) at four different times (T0, T1, T2, and T3); compare the contamination levels between the two groups at each moment; and compare the contamination levels between the four moments in each group.
Results
All pouches tested passed the reusability inspection, and none of the samples was discarded based on the exclusion criteria. Table 1 presents the results obtained after assessing the nutrient-agar Petri dishes containing the gauze dressings from the groups assayed (EG, NCG, and PCG) and for the diferente sample storage periods. As observed in Figure 2, the EG specimens showed no sign of contamination (≅ 0% microbial contamination), even after 5 months of the experiment, similar to the NCG (≅ 0% microbial contamination) and contrary to the PCG (≅ 100% microbial contamination).
�
Table 1. Presence or absence of contamination in each test group

�

�
The PCG was intentionally contaminated by exposure to a normal microbiology laboratory environment �the same environment where EG and NCG specimens were stored� and showed extensive microbial growth in all samples after the tested period. On the contrary, both EG and NCG specimens remained sterile over the storage time.
Discussion
This preliminary exploratory study focused on evaluating the reuse of sterilization paper/plastic pouches commonly used as packaging material in the healthcare sector. The results from this study show that sterilization pouches can be used a second time while maintaining sterility and integrity compared to once-used sterilization pouches, even for extended periods (153 days � 5 months of storage).
Avoiding cross-infection is a high priority in the healthcare sector for patients� safety, and, evidently, there should be no financial or material barriers to preventing the risk of healthcare-acquired infection.5, 6 However, nowadays, healthcare waste is causing significant environmental contamination from single-use plastics, which in turn, harms human health.11 - 14 Knowing that infection control is critical, this study wanted to provide a solution that would be safe and environmentally friendly.
In the studied conditions, once-used pouches (NCG) and twice-used pouches (EG) kept sterility and integrity conditions for 5 months, showing that they can be reused at least once.
The PCG samples presented microbial contamination after the whole storage period, reinforcing the storage conditions in a microbiologically contained environment. It should be noted that all sample groups were stored in the same conditions and, thus, would be prone to contamination if the pouches were compromised in terms of integrity and sterility.
The results of the present work are in line with recent investigations presented by Puangsa-Ard et al.23 In both studies, no microbial contamination was found in either the twice-used or the once-used pouches, and all remained sterile for up to 6 months. However, those studies� settings differ from the present ones. This study was designed and conducted to recreate the daily environment of a dental practice, considering the opinion of researchers that proper storage is crucial to maintain sterility and that the storage environment conditions are a more relevant factor than the type of packaging material.24, 25 Thus, sterilization pouches were stored in an environment similar to a clinical practice where samples were more susceptible to microbial contamination.
Moreover, due to the possibility of damage imperceptible to the human eye, the pouches used in this study were subjected to some manipulation, making them more prone to event-related contamination. However, the material used in this assay �gauze� is less prone to compromise their reuse, unlike sharp and rough materials. These findings indicated that reusing paper/plastic sterilization pouches could be a great start on this adjustment to a more sustainable and environmental practice.
It should be emphasized that pouches must meet minimal criteria to undergo a second sterilization cycle, which requires special care. Thus, reusing paper/plastic pouches implies special handling/procedures and careful, thorough reusability inspection by healthcare workers to guarantee their sterility and reusability.
Future investigations should repeat the experiment and do other more robust microbiological tests. Because this was a preliminary experimental scientific research aimed at providing a solution that would be safe in every procedure performed in healthcare facilities, all colony-forming units visible on the Petri dishes were classified as contamination, regardless of the number of colonies. It would also be appropriate to use two types of medium and/or broth or specific/selective culture media for double-checking contamination in the future,26 use a larger sample size, and place medical or dental instruments inside the sterilization pouches. Further research should also determine the breaking point of how many times these sterilization pouches can be reused and how long they can preserve the sterile environment.
Conclusions
Based on our study, sterilization pouches can be used a second time while maintaining sterility conditions even for extended periods.�
References
1. Oosthuysen J, Potgieter E, Fossey A. Compliance with infection prevention and control in oral health-care facilities: A global perspective. Int Dent J. 2014;64:297�311.
2. Debabrata Basu SB, Aseem Mahajan, Venkata Raman Ramanan, Mammen Chandy. Sterilization Indicators in Central Sterile Supply Department: Quality Assurance and Cost Implications. Infect Control Hosp Epidemiol. 2015;36:484-6.
3. Laneve E, Raddato B, Dioguardi M, Di Gioia G, Troiano G, Lo Muzio L. Sterilisation in Dentistry: A Review of the Literature. Int J Dent. 2019:6507286.
4. Rustagi N, Pradhan SK, Singh R. Public health impact of plastics: An overview. Indian J Occup Environ Med. 2011;15:100-3.
5. Prata JC, da Costa JP, Lopes I, Duarte AC, Rocha-Santos T. Environmental exposure to microplastics: An overview on possible human health effects. Sci Total Environ. 2020;702:134455.
6. Halden RU. Plastics and health risks. Annu Rev Public Health. 2010;31:179-94.
7. Verma R, Vinoda KS, Papireddy M, Gowda ANS. Toxic Pollutants from Plastic Waste- A Review. Procedia Environ Sci. 2016;35:701-8.
8. Smith A, Creanor S, Hurrell D, Bagg J, McCowan M. Management of infection control in dental practice. J Hosp Infect. 2009;71:353-8.
9. Volgenant CMC, de Soet JJ. Cross-transmission in the Dental Office: Does This Make You Ill? Curr Oral Health Rep. 2018;5:221-8.
10. Kamal S, Email, Kamal Sagar, Subramony B, Chaudhary P. Infection Control in Dentistry: A Review. EAS J Dent Oral Med 2020;(1):2663-7324.
11. M. Hussain, K.P. Balsara, Nagral S. Reuse of single-use devices: Looking back, looking. Natl Med J India. 2012;25:151-5.
12. Das AK, Okita T, Enzo A, Asai A. The Ethics of the Reuse of Disposable Medical Supplies. Asian Bioeth Rev. 2020;1:103-16.
13. North EJ, Halden RU. Plastics and environmental health: the road ahead. Rev Environ Health. 2013;28:1-8. 1.
14. V�zquez-Rodr�guez I, Estany-Gestal A, Seoane-Romero J, Mora MJ, Varela-Centelles P, Santana-Mora U. Quality of cross-infection control in dental laboratories. A critical systematic review. Int J Qual Health Care. 2018;30:496-507.
15. DePaola LG, Grant LE. Summary of infection control in the Dental Office: A global prospective. Infection Control in the Dental Office. 2019;:213-6.
16. Neves C, Santos N, Mendes S. Environmental sustainability practices in Portuguese dental clinics. Rev Port Estomatol Med Dent Cir Maxilofac. 2022;63:213-20.
17. Cox KD, Covernton GA, Davies HL, Dower JF, Juanes F, Dudas SE. Human Consumption of Microplastics. Environ Sci Technol. 2019;53(12):7068-74.
18. Zahid S, Anam Rafiq, Zahra S. Ilyas M. Sterilization & cross infection in dentistry; patients perception. Professional Med J 2018; 25(4):557-61.
19. Puangsa-Ard Y, Thaweboon S, Jantaratnotai N, Pachimsawat P. Effects of resterilization and storage time on sterility of paper/plastic pouches. Eur J Dent. 2018;12:417-21.
20. Campanale C, Massarelli C, Savino I, Locaputo V, Uricchio VF. A Detailed Review Study on Potential Effects of Microplastics and Additives of Concern on Human Health. Int J Environ Res Public Health. 2020;17.
21. Barker CS, Soro V, Dymock D, Fulford M, Sandy JR, Ireland AJ. Time-dependent recontamination rates of sterilised dental instruments. Br Dent J. 2011;211:E17.
22. Futo M, �iroki T, Koska S, Čorak N, Tu�ar A, Domazet-Lo�o M, et al. A novel time-lapse imaging method for studying developing bacterial biofilms. Sci Rep 12. 2022;12:21120.
23. Klumdeth J, Jantaratnotai N, Thaweboon S, Pachimsawat P. Sterility maintenance of reused disposable paper/plastic sterilization pouches in actual clinical practice. Heliyon. 2020;6:e03672.
24. McAuley T. Specifications for temperature and humidity in sterile storage environments � Where�s the evidence? Healthcare infection. 2009;14:131-7.
25. Luqueta GR, Santos EDd, Pessoa RS, Maciel HS. Evaluation of disposable medical device packaging materials under ozone sterilization. Res Biomed Eng. 2017;33:58-68.
26. Camilleri J, Arias Moliz T, Bettencourt A, Costa J, Martins F, Rabadijeva D, Rodriguez D, Visai L, Combes C, Farrugia C, Koidis P, Neves C. Standardization of antimicrobial testing of dental devices. Dent Mater. 2020;36:e59-e73.
�
Catarina Amaral
E-mail address: caprf@hotmail.com<
�
Conflict of interest
The authors have no conflicts of interest to declare.
�
Ethical disclosures
Protection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of data. The authors declare that no patient data appear in this article.
Right to privacy and informed consent. The authors declare that no patient data appear in this article.
�
CRediT authorship contribution statement
Catarina Amaral: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing � original draft. Mariana Henriques: Conceptualization, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing � review & editing. Fernanda Gomes: Conceptualization, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing � review & editing. Pedro Mesquita: Conceptualization, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing � review & editing. Maria Jo�o Ponces: Conceptualization, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing � review & editing.
�
1646-2890/� 2023 Sociedade Portuguesa de Estomatologia e Medicina Dent�ria. Published by SPEMD.
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
