
Revista Portuguesa de Estomatologia, Medicina Dentária e Cirurgia Maxilofacial
Revista Portuguesa de Estomatologia Medicina Dentária e Cirurgia Maxilofacial | 2022 | 63 (1) | 12-19
Original research
Preemptive analgesia in third molar surgery: A randomized clinical trial comparing two multimodal associations
Analgesia pré-operatória em cirurgia de terceiro molar: Um ensaio clínico randomizado comparando duas associações multimodais
a Graduate Program in Health and Society of the State University of Rio Grande do Norte, Mossoró-RN, Brazil
b Dentistry course at the State University of Rio Grande do Norte, Caicó-RN, Brazil
Erasmo Freitas de Souza Junior - erasmo_jn@hotmail.com
Article Info
Rev Port Estomatol Med Dent Cir Maxilofac
Volume - 63
Issue - 1
Original research
Pages - 12-19
Go to Volume
Article History
Received on 15/03/2021
Accepted on 18/12/2021
Available Online on 21/01/2022
Keywords
Original Research
�
Preemptive analgesia in third molar surgery: A randomized clinical trial comparing two multimodal associations
Analgesia pr�-operat�ria em cirurgia de terceiro molar: Um ensaio cl�nico randomizado comparando duas associa��es multimodais
�
Erasmo Freitas de Souza Junior1,* 0000-0003-1714-9226
Jos� Iago Pereira de Brito2 0000-0003-1802-8485
Janielma Azevedo Silva1 0000-0002-5374-4324
Cinthia Mayara Rodrigues Xavier1 0000-0002-1184-2911
Eudes Euler de Souza Lucena1 0000-0003-3119-7822
H�cio Henrique Ara�jo de Morais1 0000-0002-6450-1483
1 Graduate Program in Health and Society of the State University of Rio Grande do Norte, Mossor�-RN, Brazil
2 Dentistry course at the State University of Rio Grande do Norte, Caic�-RN, Brazil.
�
�
Article history:
Received 15 March 2021
Accepted 18 December 2021
Available online 24 January 2022
�
Abstract
Objective: This study aimed to verify the effect of preemptive administration of dexamethasone 8 mg co-administered with paracetamol 1 g compared with dexamethasone 8 mg co-administered with nimesulide 100 mg in surgeries for extracting third molars.
Methods: A prospective, randomized, triple-blind clinical trial was conducted, allocating patients into two groups by the split-mouth method: Group 1 received dexamethasone and paracetamol, and Group 2 received dexamethasone and nimesulide. Each patient underwent two surgeries on different occasions, evaluating the parameters: pain, number of consumed rescue analgesics, time to the first rescue analgesic consumption, edema, trismus, and patient satisfaction.
Results: Similar results were found in pain, trismus, number of rescue analgesics ingested, time until ingesting the first rescue analgesic, and overall assessment variables. However, Group 1 showed better results regarding edema, with a statistically significant difference in the 48-h period (p=0.028).
Conclusion: Dexamethasone 8 mg plus paracetamol 1 g is effective for preemptive analgesia.
Keywords: Anti-inflammatory agents,Edema, Pain, Third molar,Trismus
�
Resumo
Objetivo: Este estudo verificou o efeito da administra��o pr�-operat�ria da dexametasona 8mg coadministrada com paracetamol 1 g em compara��o com a dexametasona 8 mg coadministrada com nimesulida 100 mg em cirurgias de extra��o de terceiros molares.
M�todos: Foi realizado um ensaio cl�nico prospetivo, randomizado e triplo-cego, alocando os pacientes em 2 grupos pelo m�todo da boca dividida: Grupo 1 (dexametasona e paracetamol) e Grupo 2 (dexametasona e nimesulida). Cada paciente foi submetido a duas cirurgias em ocasi�es diferentes, avaliando os par�metros: dor, n�mero de analg�sicos de resgate consumidos, tempo para o primeiro consumo do analg�sico de resgate, edema, trismo e satisfa��o do paciente.
Resultados: Foram encontrados resultados semelhantes para dor, trismo, n�mero de analg�sicos de resgate ingeridos, tempo necess�rio para a ingest�o do primeiro analg�sico de resgate e avalia��o global. Por�m, o Grupo 1 apresentou melhores resultados em rela��o ao edema, com diferen�a estatisticamente significativa no per�odo de 48h (p=0,028).
Conclus�o: Dexametasona 8 mg mais paracetamol 1 g � uma possibilidade efetiva para a analgesia pr�-operat�ria.
Palavras-chave: Agentes anti-inflamat�rios, Edema, Dor,Terceiro molar, Trismo
�
Introduction
Surgeries for third molar removal and other elements thereof cause significant tissue trauma and generate inflammation, pain, edema, and trismus.1 - 9 Preemptive analgesia (PA) has developed as a technique that seeks to initiate anti-inflammatory and analgesic effects using pre-surgery drugs before painful stimulation. This approach prevents hyperalgesia and provides a better quality of life in the post-surgery period, principally faster recovery and earlier return to daily activities.(1, 2, 6, 8, 10 - 14) Several pharmacological methods for PA have been described, such as local anesthetics, corticosteroids, nonsteroidal anti-inflammatory drugs (NSAIDs), analgesics, and combinations of these drugs. 5, 6, 9, 10, 14
Regarding the administration of anti-inflammatory drugs, studies have shown that preemptively administered dexamethasone (generally 8 mg) reflects on less post-surgery pain and less consumption of rescue analgesics and a longer time to resort to them.6,15 These advantages result from those drugs inhibiting the action of the phospholipase A2 enzyme, which breaks down the remaining inflammation chain. 8 In addition, NSAIDs are excellent drugs for dental pain control due to being able to limit peripheral sensitization and reduce the synthesis of prostaglandins on the surgical site by inhibiting the cyclooxygenase (COX) enzymes; this results in a positive response to its pre-surgery use. 2 - 4, 11 Furthermore, better antalgic effects have been observed in the multimode association of 8 mg dexamethasone with 100 mg nimesulide compared with 8 mg dexamethasone alone in a randomized study. 7 Nevertheless, these drugs may cause adverse effects, such as nausea, vomiting, gastritis, ulcers, gastrointestinal hemorrhage, and increased trans and post-surgery bleeding. Thus, this combination would not be indicated for a portion of the population, i.e., patients already predisposed for such disorders. 3, 10, 16 On the other hand, paracetamol is effective and has an excellent safety profile as an analgesic, i.e., it does not show the aforementioned side effects.9, 16, 17
PA is still a controversial topic and requires further study. 2, 5, 10, 12 Therefore, this study aims to contribute to the improvement of this technique by comparing the coadministration of 1 g paracetamol with 8 mg dexamethasone versus the coadministration of 8 mg dexamethasone with 100 mg nimesulide in the control of post-surgery pain, edema, and trismus.
Material and methods
This study was submitted to and approved by the Research Ethics Committee of the State University of Rio Grande do Norte (UERN), Brazil. It is also compliant with the Declaration of Helsinki.
The inclusion criteria were volunteer patients from spontaneous demand in the age group between 16 and 45 years old who attended the facilities of the Dentistry Course of UERN and agreed to participate in the research after reading the Free and Informed Consent Form. Patients were classified according to the American Society of Anesthesiology (ASA) as an ASA I group (normally healthy, no systemic alterations, and no continuous use of drugs)18with the indication of bilateral lower third molar extraction. The teeth had mandatorily the same impaction degree and inclination as their contralateral per Pell & Gregory�s classification and Winter�s classification, respectively.19 The exclusion criteria were as follows: reporting unpleasant previous experiences (allergic reactions or processes) with any of the drugs used in this research; reporting using concomitant medication; not returning for the second surgery (i.e., on the opposite side); not returning for the evaluations; not being able to answer the questionnaires; and not filling out the evaluation forms correctly.
This study was an analytical split-mouth clinical trial. Patients were allocated randomly to two groups through a raffle. The study was triple-blind, i.e., patients, operators, and evaluators did not know the type of medication used in each case. The sample size was defined based on data collection from 10 patients with the Bioestat 5.0 computer program using a bilateral test that took into account data obtained from the pain variable in a 6-hour period, with a test power of 95% and a significance level of 5%. The difference between means (0.6) and standard deviation (0.64) of the pilot sample was considered for calculation, which resulted in 17 patients. A total of 22 patients (approximately 30% more than the 17 patients) were used as a strategy to compensate for possible losses that could occur during data collection.
The randomization procedure was performed by a research team member that did not participate in the surgeries and statistical analyses. Two groups of opaque and sealed envelopes were used: one with the combination of the drug to be administered, i.e., dexamethasone 8 mg plus paracetamol 1 g (Group 1) or dexamethasone 8 mg plus nimesulide 100 mg (Group 2), identified as A and B, and the other with the surgery side (right or left). There was parity in the number of envelopes indicating the drug combination, so half of the patients started the surgical procedures with one combination and the other half with the opposing combination. The second surgery was performed on the contralateral side with the administration of the second combination of drugs.
To avoid disclosing which drug combination was administered in each surgery, paracetamol and nimesulide were manipulated in a compounding pharmacy packed in bottles of the same color and size. Only those responsible for the pharmacy (not participants in the research) were aware of which drug was in each bottle, and the research team did not know which was drug A and drug B until the conclusion of the statistical analyses. Dexamethasone was also compounded in the same pharmacy, but masking was unnecessary since both groups would use it.
Patients underwent a mouthwash with 0.12% chlorhexidine for one minute as pre-surgery medication, as well as topical, extraoral 2% chlorhexidine friction with a sterile gauze. Then, the research drugs, dexamethasone (8 mg, one capsule) plus paracetamol (1 g, two 500 mg capsules) or dexamethasone (8 mg, one capsule) plus nimesulide (100 mg, two 50 mg capsules), were administered orally 1 hour before starting the surgical procedure. As a post-surgery prescription, patients were medicated orally with one 500-milligram tablet of sodium dipyrone and instructed to take another when they felt discomfort corresponding to a score of 3 or higher in the visual analog scale (VAS), always respecting the 6-hour minimum interval between tablets. The VAS is a rule-shaped scale ranging from 0 to 10, where 0 is the absence of pain and 10 is the maximum pain; scores 0�2 are considered mild, 3�7 moderate, and 8�10 intense pain. Chlorhexidine-based collutory was prescribed at 0.12% twice a day for seven days, as it is useful for cleaning the surgical site and controlling halitosis. Amoxicillin 500 mg was also prescribed every 8 hours for seven days.
Demographic characteristics (gender, age, race, weight, height, and impaction classification) were recorded, as well as the following: surgical time (from soft tissue incision to the end of the suture procedure), number of anesthetic tubes administered, accidents, and complications. The dependent variables studied were pain, number of ingested rescue analgesics, time until the first rescue analgesic consumption, edema, trismus, and patient satisfaction (by overall assessment).
The pain variable was quantified through VAS, asking the patient to indicate the intensity of their pain in the first 30 minutes after surgery and after 2, 4, 6, 8, 12, 16, 24, 48, and 72 hours. The total number of rescue analgesics consumed until the seventh post-surgery day and the exact time when these were ingested were also recorded. The overall assessment is amethod in which patients classify their satisfaction with the applied therapeutics using a 5-point Likert scale (0, bad; 1, reasonable; 2, good; 3, very good; and 4, excellent).
The edema was evaluated with a measuring tape, with three measurements taken in patients using three distances between five reference points: (A) eye corner/jaw angle; (B) tragus/mouth corner; (C) tragus/pogonion. Measurements were performed pre-surgery and after 24, 48, and 72 h, and 7 days after surgery (Figure 1). The edema evolution was obtained by subtracting the total value of each post-surgery time point (mean of the three distances) from the mean of the pre-surgery measurements.
�
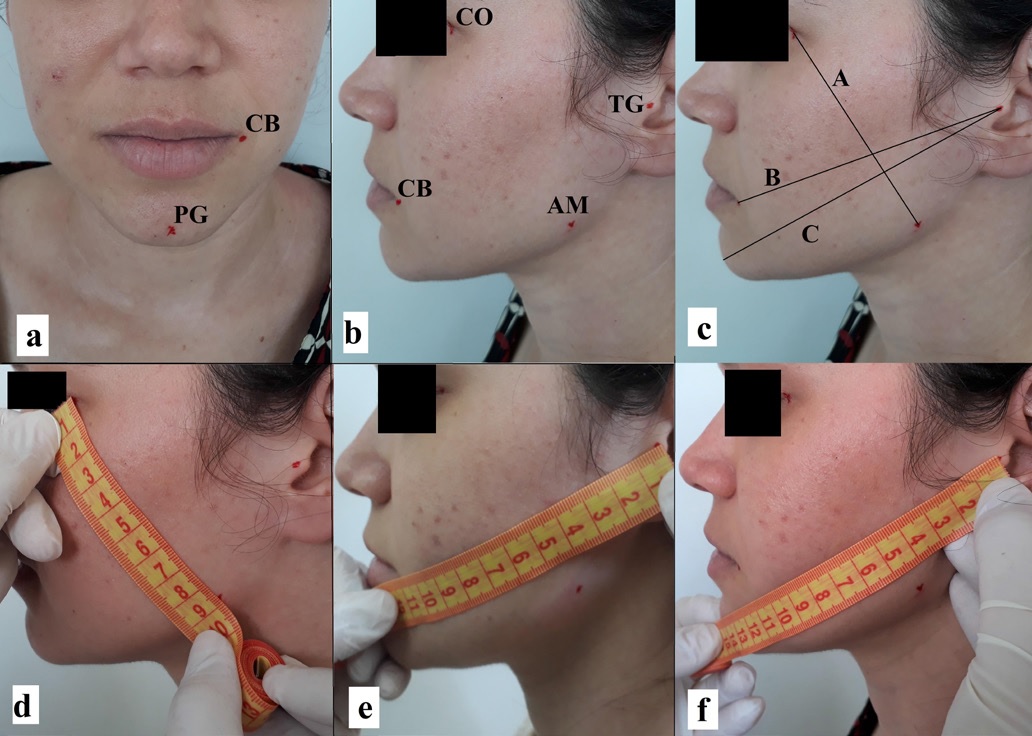
Figure 1. Measurement of edema. a: facial points pogonion (PG) and mouth corner (CB); b: facial points eye corner (CO), mouth corner (CB), tragus (TG), and mandible angle (AM); c: distance A (CO to AM), distance B (TG to CB), and distance C (TG to PG); d: distance measurement A; e: distance measurement B; f: distance measurement C.
�
The trismus was measured by caliper based on the maximum mouth opening between the upper and lower central incisors (Figure 2). Measurements were performed pre-surgery and after 24, 48, and 72 h, and 7 days. This variable was determined by the difference between the pre-surgery measurement and each post-surgery measurement.
�
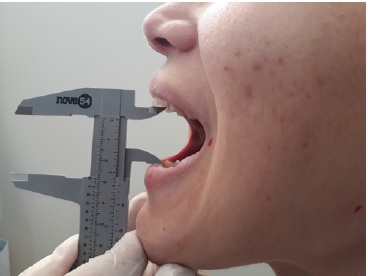
Figure 2. Measurement of trismus by maximum mouth opening.
�
In the statistical analysis, normality was verified for all variables, considering normal distribution when the following three items were fulfilled: variation coefficient < 50%; asymmetry and kurtosis smaller than two times their respective standard errors; and all data of each variable within the interval between the average minus three times the standard deviation and the average plus three times the standard deviation.
After that analysis, the Wilcoxon test was used to compare the results of the variables. A significance level of 5% was adopted. The p-values smaller than 0.05 were considered statistically significant.
Results
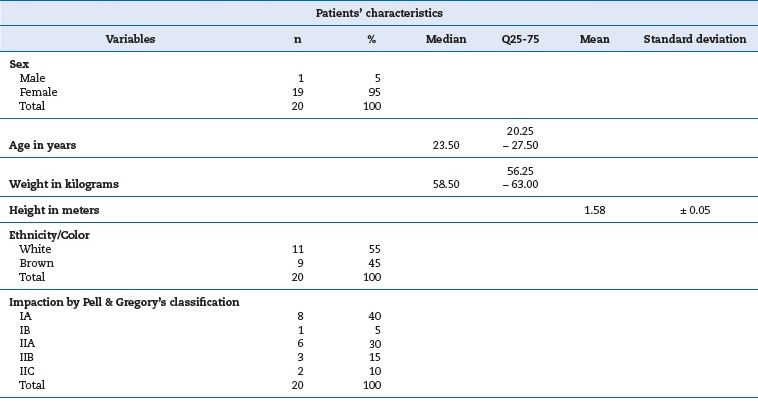
The recruitment of patients occurred from June 20, 2017, to March 29, 2019. Twenty-three patients fit the inclusion criteria, but three were excluded due to not returning the post-surgery assessments of the first surgery. Thus, twenty patients concluded all research steps, exceeding by three the figure predetermined by sample calculation. The patients were allocated into groups (Figure 3). Table 1 summarizes the patients� characteristics.
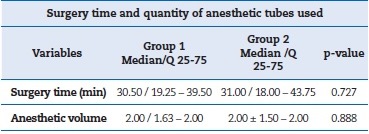
Regarding surgery time and the number of anesthetic tubes used in each moment, the Wilcoxon test showed a p-value greater than 0.05, so the differences were not significant, and such factors did not interfere with the research results.
�
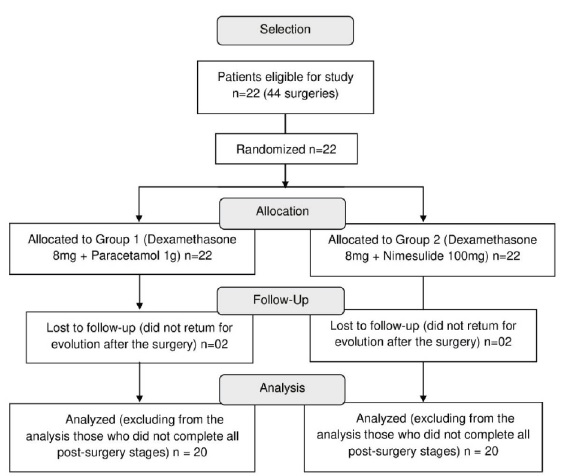
Figure 3. Study flowchart
�
Table 1. Patients� characteristics expressed as median and interquartile range (age, weight), arithmetic means and standard deviations (height), and percentages (sex, ethnicity/color, and impaction degrees).
�
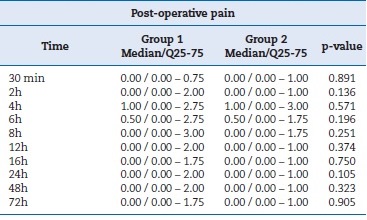
Therefore, these items were not considered confounding variables. Table 2 summarizes the values for surgery time and anesthetic tubes used. For the VAS-verified pain intensity variable, the Wilcoxon test showed a p-value greater than 0.05 for all time points, as shown in Table 3. Therefore, the differences were not statistically significant.
�
Table 2. Surgery time and anesthetic volume variables expressed as median, interquartile range and p-value by Wilcoxon test.
�
Table 3. Pain variable by the visual analog scale expressed as median, interquartile range, and p-value.
�
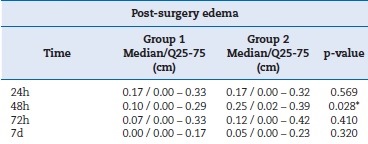
When comparing post-surgery edema between the groups, the Wilcoxon test showed a p-value greater than 0.05 in the 24h, 72 h, and 7 days time points. In turn, the p-value was smaller than 0.05 (p=0.028) for the 48 h time point, showing a statistically significant difference in favor of the association of paracetamol and dexamethasone. Table 4 and Figure 4 summarize the values for edema.
�
Table 4. Post-surgery edema variable expressed as median, interquartile range, and p-value.
�
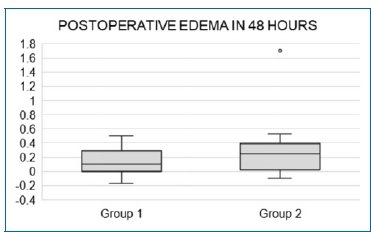
Figure 4. Boxplot comparing the edema value at 48 h; a discrepant value is observed in the disfavor of Group 2.
�
Concerning post-surgery mouth opening, the Wilcoxon test showed a p-value greater than 0.05 between the groups for all time points. Therefore, the differences were not statistically significant. Table 5 summarizes the values for limitation in mouth opening.
Regarding the time until resorting to the first rescue analgesic tablet and the number of rescue analgesics ingested, the Wilcoxon test showed a p-value greater than 0.05 for both cases. Table 6 summarizes the values for rescue analgesics.
�
Table 5. Mouth opening limitation variable expressed as median, interquartile range, and p-value by Wilcoxon test.
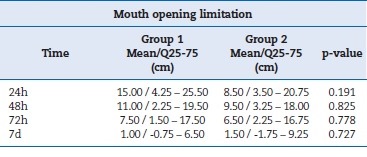
�
Table 6. Number of rescue analgesics ingested and time until the first rescue analgesic tablet expressed as median, interquartile range, and p-value by Wilcoxon test.
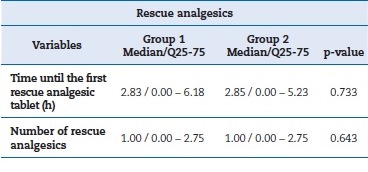
�
Both groups presented satisfactory values in the overall assessment, and the Wilcoxon test showed a p-value greater than 0.05 between the global satisfaction averages for both groups.
Therefore, the differences were not statistically significant. Table 7 summarizes the values for the overall assessment.
�
Table 7. Overall assessment expressed as median, interquartile range, and p-value by Wilcoxon test.
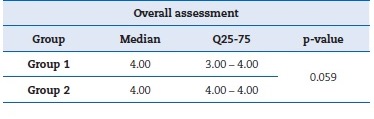
�
Discussion
The fact that PA reduces complications and the need for post-operatory analgesics has been consolidated in the global literature. However, the best drug and/or association is still uncertain. In this scenario, dexamethasone, nimesulide, and paracetamol have been studied and compared with other drugs in various research formats in PA for third molar extraction, including multimodal associations. The comparison of these studies can guide the selection of drugs.1, 7, 9, 10, 14, 16, 20, 21 It is valid to emphasize that clinical studies are necessary to assess the effectiveness of PA. In that regard, third molar extraction is a commonly used study model to test the performance of analgesics and anti-inflammatory drugs, principally by the split-mouth method. However, a potentially negative pain experienced in the first surgical moment may affect the patient�s perception regarding pain sensation in a further surgical moment; this may become a limitation in this type of study.1, 16, 20 To overcome this limitation in the present research, greater care was taken in the randomization process by assuring parity when indicating the drug association to be administered in the first surgery. Such care allowed minimizing this confusion bias.
The studies involving dexamethasone have used it compared with ketorolac;1 combined with tramadol in comparison to the association of tramadol with sodium diclofenac;14 combined with ibuprofen versus isolated administration of ibuprofen;20 and combined with nimesulide versus isolated administration of dexamethasone.7 In all these situations, the groups containing dexamethasone were more effective. The studies also pointed out a better response when dexamethasone was administrated concomitantly with another anti-inflammatory medicine, as in the present study. Furthermore, most of those studies used an 8 mg concentration of dexamethasone, which is aligned with a meta-analysis that pointed it as the dosage that promotes the best results when using this drug for PA in third molar extraction.6
Nimesulide 100 mg has also been investigated regarding its use before third molar extraction, namely, compared with tramadol 100 mg21 or administered in a multimodal way with 8 mg dexamethasone versus isolated use of 8 mg dexamethasone,7 where the groups containing nimesulide presented a better response. On the other hand, 500 mg paracetamol was used associated with 30 mg codeine versus placebo in the control group and presented a better, statistically significant response for the variables pain and time for the ingestion of the first rescue analgesic dose.9 It was also studied in the concentration of 1 g paracetamol associated with 30 mg codeine and presented better results than the isolated administration of 1g paracetamol for pain relief after the surgical removal of retained third molars, which reinforces the benefits of the combination of medicines for this purpose.16
Based on the aforementioned studies, dexamethasone can be considered the medication of choice in PA, principally with the concentration of 8 mg. However, a multimodal association with other medicines, namely nimesulide and paracetamol, is plausible and tends to yield a better response. The results of the present study showed that the dexamethasone plus nimesulide and dexamethasone plus paracetamol associations presented a good therapeutic response for the majority of the studied variables. However, the group using paracetamol showed a better response to edema, with a statistically significant difference for the 48 h time point (p=0.028). Therefore, this medicine combination may be preferable in daily clinical management for third molar extraction because it presented a similar or better response than the nimesulide association and is less deleterious to the gastrointestinal tract.9, 16, 17
Regarding the hepatotoxic effect attributed to both nimesulide and paracetamol, there is no evidence that a single therapeutic dose, as used in the present research, can cause an expected hepatotoxic event, principally in young adult patients, who are generally those indicated for third molar extraction. 22, 23 Besides, better preemptive control of the inflammatory symptomatology tends to diminish the additional, more prolonged use of anti-inflammatory/analgesic medication in post-surgery, as occurred in the present research, which can be considered a protective factor for problems of this nature.
In the present research, there was no adverse event in any patient.
Conclusions
The coadministration of 8 mg dexamethasone plus 1 g paracetamol presented a response similar to the combination of dexamethasone 8 mg plus 100 mg nimesulide in the variables pain, lockjaw, number of rescue analgesics ingested, time until the ingestion of the first rescue analgesic, and overall assessment, with no statistically significant differences. However, post-surgery edema had a statistically significantly better response in a 48-h timespan in the group containing paracetamol.
Therefore, 8 mg dexamethasone plus 1 g paracetamol can be a plausible option in clinical management.
�
References
1 Paiva-Oliveira JG, Bastos PRHO, Pontes ERJC, Silva JCL, Delgado JAB, Oshiro-Filho, NT. Comparison of the anti-inflammatory effect of dexamethasone and ketorolac in the extractions of third molars. Oral Maxillofac Surg. 2016;20:123-33.
2 Orozco-Solis M, Garcia-Avalos Y, Pichardo-Ramirez C, Tobias-Azua F, Zapata-Morales J, Aragon-Martinez O, et al. Single dose of diclofenac or meloxicam for control of pain, facial swelling, and trismus in oral surgery. Med Oral Patol Oral Cir Bucal. 2016;21:127-34.
3 Mishra H, Khan F. A double-blind, placebo-controlled randomized comparison of pre and postoperative administration of ketorolac and tramadol for dental extraction pain. J Anaesthesiol Clin Pharmacol. 2012;28:221-5.
4 Mony D, Kulkarni D, Shetty L. Comparative Evaluation of Preemptive Analgesic Effect of Injected Intramuscular Diclofenac and Ketorolac after Third Molar Surgery- A Randomized Controlled Trial. J Clin Diagn Res. 2016;10:102-6.
5 Espinoza MAI, Pozos-Guillen A, Martinez-Rider R, Perez-Urizar J. Comparison of the analgesic efficacy of oral ketorolac versus intramuscular tramadol after third molar surgery : A parallel, double-blind, randomized, placebocontrolled clinical trial. Med Oral Patol Oral Cir Bucal. 2016;21:637-43.
6 Falci, SGM, Lima TC, Martins CC, Santos CRR, Pinheiro MLP. Preemptive Effect of Dexamethasone in Third-Molar Surgery : A Meta-Analysis. Anesth Prog. 2017;64:136-43.
7 Barbalho JC, Vasconcellos RJH, Morais HH, Santos LAM, Almeida RAC, R�belo HL, et al. Effects of co-administered dexamethasone and nimesulide on pain, swelling, and trismus following third molar surgery: a randomized, triple-blind, controlled clinical trial. Int J Oral Maxillofac Surg. 2017;46:236-42.
8 Lima TC, Bagordakis E, Falci SGM, Santos CRR, Pinheiro MLP. Pre-emptive effect of dexamethasone and diclofenac sodium associated with codeine on pain, swelling, and trismus after third molar surgery: a split-mouth randomized triple-blind controlled clinical trial. J Oral Maxillofac Surg. 2018;76:60-6.
9 Cristalli MP, Monaca GL, Angelis C, Pranno, N, Annibali S.Efficacy of Preoperative Administration of Paracetamol-Codeine on Pain following Impacted Mandibular Third Molar Surgery : A Randomized, Split-Mouth, Placebo-Controlled, Double-Blind Clinical Trial. Pain Res Manag. 2017;2017:9246352.
10 Costa FWG, Esses DFS, Silva PGB, Carvalho FSR, S� CDL, Albuquerque AFM, et al. Does the Preemptive Use of Oral Nonsteroidal Anti-inflammatory Drugs Reduce Postoperative Pain in Surgical Removal of Third Molars ? A Meta-analysis of Randomized Clinical Trials. Anesth Prog. 2015;62:57-63.
11 Shah AV, Kumar KVA, Rai KK, Kumar BPR. Comparative Evaluation of Pre-Emptive Analgesic Efficacy of Intramuscular Ketorolac Versus Tramadol Following Third Molar Surgery. J Maxillofac Oral Surg. 2013;12:197-202.
12 Liporaci Junior JL. Assessment of Preemptive Analgesia Efficacy in Surgical Extraction of Third Molars. Rev Bras Anestesiol. 2012;62:502-10.
13 Waldron NH, Jones CA, Gan TJ, Allen TK, Habib AS. Impact of perioperative dexamethasone on postoperative analgesia and side-effects : systematic review and meta-analysis. Br J Anaesth. 2013;110:191-200.
14 Santos JASS, Silva LCF, Santos TS, Menezes J�nior LR, Oliveira ACA, Brand�o JRMCB. Comparative study of tramadol combined with dexamethasone and diclofenac sodium in third-molar surgery. J Craniomaxillofac Surg. 2012;40:694-700.
15 Almeida RAC, Lemos CAA, de Moraes SLD, Pellizzer EP, Vasconcelos BC. Efficacy of corticosteroids versus placebo in impacted third molar surgery: systematic review and meta-analysis of randomized controlled trials. Int J Oral Maxillofac Surg. 2019;48:118-31.
16 Macleod AG, Ashford B, Voltz M, Williams B, Cramond T, Gorta L, et al. Paracetamol versus paracetamol-codeine in the treatment of post-operative dental pain : A randomized, double-blind, prospective trial. Aust Dent J. 2002;47:147-51.
17 Caliskan E, Sener M, Kipri M, Yilmaz I, Aribogan A. Comparison of the effects of intravenous Dexketoprofen Trometamol versus Paracetamol on postoperative analgesia in patients undergoing Septoplasty : A randomised doubleblind clinical trial. Pak J Med Sci. 2018;34:546-52.
18 Hackett NJ, De Oliveira GS, Jain UK, Kim JY. ASA class is a reliable independent predictor of medical complications and mortality following surgery. Int J Surg. 2015;18:184-90.
19 Park K. Which factors are associated with difficult surgical extraction of impacted lower third molars? J Coreano Assoc Oral Maxillofac Surg. 2016;42:251-8.
20 Bauer HC, Duarte FL, Horliana ACRT, Tortamano IP, Perez FEG, Simone JL, et al. Assessment of preemptive analgesia with ibuprofen coadministered or not with dexamethasone in third molar surgery : a randomized double-blind controlled clinical trial. Oral Maxillofac Surg. 2013;17:165-71.
21 Ara�jo FAC, Santos TS, Morais HHA, Laureano Filho JR, Silva EDO, Vasconcellos RJH. Comparative analysis of preemptive analgesic effect of tramadol chlorhydrate and nimesulide following third molar surgery. J Craniomaxillofac Surg. 2012;40:346-9.
22 Acheampong P, Thomas SH. Determinants of hepatotoxicity after repeated supratherapeutic paracetamol ingestion: systematic review of reported cases. Br J Clin Pharmacol. 2016;82:923-31.
23 Kwon J, Kim S, Yoo H, Lee E. Nimesulide-induced hepatotoxicity: A systematic review and meta-analysis. PLoS One. 2019;14:1-18.
�
Erasmo Freitas de Souza Junior
E-mail address: erasmo_jn@hotmail.com
�
Ethical disclosures
Protection of human and animal subjects. The authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of data. The authors declare that they have followed their work center protocols on access to patient data and for its publication.
Right to privacy and informed consent. The authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
�
Conflict of interest
The authors have no conflicts of interest to declare.
�
1646-2890/� 2022 Sociedade Portuguesa de Estomatologia e Medicina Dent�ria. Published by SPEMD.
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
