
Revista Portuguesa de Estomatologia, Medicina Dentária e Cirurgia Maxilofacial
Revista Portuguesa de Estomatologia Medicina Dentária e Cirurgia Maxilofacial | 2021 | 62 (1) | 29-34
Original research
Lichen planus and its therapeutic management: a retrospective study
Líquen Plano e abordagem terapêutica: um estudo retrospetivo
a Faculdade de Medicina Dentária, Universidade de Lisboa, Lisbon, Portugal
b Centro de Estatística e Aplicações, Universidade de Lisboa, Lisbon Portugal
c Escola Superior de Tecnologia e Gestão do Instituto Politécnico de Leiria, Leiria, Portugal
d Faculdade de Medicina, Universidade de Lisboa, Lisbon, Portugal
Mariana Rodrigues Rebelo - rebelo.mariana@campus.ul.pt
Article Info
Rev Port Estomatol Med Dent Cir Maxilofac
Volume - 62
Issue - 1
Original research
Pages - 29-34
Go to Volume
Article History
Received on 28/08/2020
Accepted on 21/02/2021
Available Online on 25/03/2021
Keywords
Original Research
�
Lichen planus and its therapeutic management: a retrospective study
L�quen Plano e abordagem terap�utica: um estudo retrospetivo
�
Mariana Rodrigues Rebeloa,*, Cristina Palmela Pereiraa,b, Rui Sousa Santosb,c, Lu�s Soares-de-Almeidad, Paulo Filiped
a Faculdade de Medicina Dent�ria, Universidade de Lisboa, Lisbon, Portugal.
b Centro de Estat�stica e Aplica��es, Universidade de Lisboa, Lisbon Portugal.
c Escola Superior de Tecnologia e Gest�o do Instituto Polit�cnico de Leiria, Leiria, Portugal.
d Faculdade de Medicina, Universidade de Lisboa, Lisbon, Portugal.�
�
Article history:
Received 28 August 2020
Accepted 20 February 2021
Available online xx March 2021
�
Abstract
Objectives: To study the epidemiological distribution of the disease lichen planus and its therapeutic management.
Methods: A total of 174 patients with a lichen planus diagnosis between 2008 and 2017 at a Portuguese public hospital were included in this retrospective study. The following data were collected from clinical records: gender, age, topographic distribution of lesions, prescribed therapy (active substance, route, and scheme of administration), mean duration of illness, and episodes of cure and relapse. Statistical analysis was performed using IBMR SPSSR Statistics software, version 25.
Results: In this population, lichen planus affected both genders with the same probability (p=0.820), and was more prevalent in the 4th-5th decades of age. The lesions appeared in the skin (75.9% of the patients), mucous membranes (5.2%), or both (19.0%). The most prescribed drugs were corticosteroids, followed by antihistamines and immunosuppressants. Topical corticosteroids were the most common ones, namely clobetasol propionate (37.4%). Within the systemic corticosteroids, prednisolone was the most prescribed drug (12.3%). The average duration of lesions and symptoms was approximately 6.5 months. For the relapsing population (12%), the mean period of symptoms� remission was 513 days.
Conclusions: The epidemiological parameters of lichen planus in these Portuguese patients bear similarities with other described populations. No evidence-based therapeutic has proven to be effective for lichen planus treatment, but topical corticosteroids continue to be the firstline therapy for this pathology.
Keywords: Corticosteroids,Dermatology,� Epidemiology,Lichen planus,Therapeutics
�
Resumo
Objetivos: Caracterizar epidemiologicamente a patologia L�quen Plano e sua abordagem terap�utica.
M�todos: Neste estudo retrospetivo, foram inclu�dos 174 pacientes, de um hospital p�blico portugu�s, com diagn�stico de L�quen Plano entre os anos 2008 e 2017. Os registos m�dicos permitiram a recolha de informa��o relativa a g�nero, idade, distribui��o topogr�fica das les�es de L�quen Plano, terap�utica prescrita (princ�pio ativo, via de administra��o e esquema terap�utico), dura��o m�dia da doen�a, epis�dios de cura e recidiva. A an�lise estat�stica foi realizada com recurso ao software IBM� SPSS� Statistics, vers�o 25.
Resultados: Nesta popula��o, a doen�a afetou ambos os g�neros com a mesma probabilidade (p=0,820), no entanto, com maior preval�ncia na 4.� e 5.� d�cadas de idade. As les�es de L�quen Plano manifestaram-se na pele (em 75,9% dos doentes), nas mucosas (5,2%), ou em ambos (19,0%). Os f�rmacos mais prescritos foram os corticoster�ides, seguidos dos anti-histam�nicos e dos imunossupressores. Os corticoster�ides t�picos foram os mais comummente administrados, nomeadamente o Propionato de Clobetasol (37,4%). Dentro dos corticoster�ides sist�micos, a Prednisolona foi o f�rmaco mais utilizado (12,3%). A dura��o m�dia das les�es e sintomas foi de aproximadamente 6,5 meses. Para a popula��o que recidivou (12%), o per�odo m�dio de remiss�o dos sintomas foi de 513 dias.
Conclus�es: Os par�metros epidemiol�gicos desta amostra de pacientes portugueses com L�quen Plano partilham semelhan�as com outras popula��es descritas. Nenhuma terap�utica baseada em evid�ncia provou ser eficaz para o tratamento do L�quen Plano. No entanto, os corticoster�ides t�picos continuam a apresentar-se como a solu��o terap�utica de primeira linha para esta patologia.
Palavras-chave: Corticoester�ides,Dermatologia, Epidemiologia,L�quen plano,Terap�utica
�
Introduction
Lichen planus (LP) is an inflammatory, immunologically mediated, chronic disease.1 - 3 It represents a mucocutaneous entity present in about 0.5 to 5% of the adult population and can affect any age or gender.4 ‑ 6
However, some studies report a preferential occurrence of LP in female individuals aged 30 to 60 years, being rare among children.4, 7, 8
Clinically, its lesions can appear in several body locations, such as the skin, nails, scalp, oral and genital mucosa, and, less frequently, ocular, tear, and gastrointestinal tract mucosa.(6 9 10) Cutaneous lichen planus (CLP) commonly manifests as small polygonal violet papules, although it can take several forms depending on the disease�s subtype.6, 8, 11 Oral lichen planus (OLP) occurs as the only manifestation of the disease in 15-25% of patients and is associated with skin lesions in 60-70% of the cases.3, 4 It has several subtypes, and the most common is the reticular one, which is usually asymptomatic.5 OLP deserves careful consideration from health professionals, as the latest studies confirm the possibility of malignancy, especially in the erosive subtype.12, 13
The etiopathogenesis of this disease is not fully clarified. However, the literature suggests the occurrence of an autoimmune process mediated by deregulated T cells that compromise basal keratinocytes of the epithelium.(4, 14, 15) Some factors were associated with this pathology, such as HCV (hepatitis C virus) or HPV (human papillomavirus) infection.3, 6, 16, 17 Moreover, the association between stress/anxiety and OLP is well established.18
As in all autoimmune diseases, LP treatment is not curative but aims to reduce/eliminate lesions and the associated symptomatology.19 Topical corticosteroids represent the first current line treatment for both CLP and OLP. However, their effectiveness has not yet been proved by studies of strong scientific evidence.1, 2, 19, 22 Thus, it is urgent to find the most favorable therapeutic regimen for better treatment of our patients.2
Therefore, this investigation aims to study the epidemiological distribution of LP and its therapeutic management in a sample of the Portuguese public healthcare population.
Material and Methods
This investigation consists of a retrospective, observational, case series study. It was carried out at the Dermatology Service of one of Portugal�s biggest public hospitals: Hospital de Santa Maria, Lisbon. This study included patients with a clinical and histological diagnosis of LP, observed from 2008 to 2017. Of 340 patients, 174 were included in this study; the remaining were excluded due to lack of information or inconclusive diagnosis. The data was blindly collected from the patients� clinical records to preserve the anonymity and confidentiality of all data. This study was approved by the Hospital Ethics Committee.
The variables under study were:
� Regarding the patient: 1) date of the appointment; 2) gender; 3) age of the 1st manifestation of LP (except when otherwise indicated, the date of the patient�s first Dermatology appointment was considered); 4) location of the LP�s lesions (cutaneous vs. oral vs. genital); 5) topographic distribution of skin lesions; 6) topographic distribution of oral lesions; 7) topographic distribution of genital lesions; 8) administration of therapy (yes/no).
� Regarding the therapeutic regimen (data not collected if no therapy was administered): 9) therapy start date; 10) pharmacological group(s) administered; 11) corticosteroid active substance (AS)* (and corresponding a) route of administration; b) pharmaceutical form; c) dose; d) daily dosage; and e) drug administration scheme); 12)
antihistamine AS (followed by data from a) to e)*); 13) immunosuppressant AS (followed by data from a) to e)*); 14) follow-up; 15) result of the established therapy; 16) mean duration of symptoms; 17) recurrence events; and 18) symptom remission period. Variables 15 to 18 were only collected if there were records of the follow‑up.
The statistical analysis was performed using the software IBMR SPSSR Statistics, version 25. Hypotheses were tested using the binomial and chi‑square tests of one sample and the Kolmogorov-Smirnov test of two independent samples.
Results
The study population consisted of 85 men (48.9%) and 89 women (51.1%), meaning that LP occurred in both genders with the same probability (p=0.820). The minimum age was 11 years old and the maximum 88. Table 1 shows the age of the first manifestation of the disease, divided by decades, reflecting that the 4th and 5th were the most affected. There were no differences in age distribution between genders (p=0.989).
�

�
Regarding the distribution of LP lesions, no statistical differences were found between trimesters (p=0.235). As shown in Table 2, the skin was largely the most affected location, either alone or with mucous membranes (n=165;94.9%). Among patients with skin lesions, 40.7% only had one site affected, 22.8% had two, 32.5% had three or more, and 4% had no site information. The most affected sites were the lower limb, the torso, the upper limb, and the extremities.
�

The topographic distribution of oral lesions is presented in Table 3. The buccal mucosa was the most affected location, representing 70% of the oral cavity lesions. There were no records of malignant transformation of OLP lesions.
�
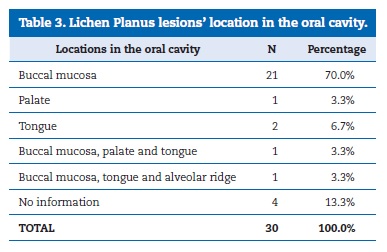
�
Regarding the therapeutic regimen, only 166 patients (95.4%) had a record of the prescribed therapeutics. Half of them had a therapeutic regimen with only one drug, while the other half had a combination of medicines. The main pharmacological groups were corticosteroids and antihistamines, followed by immunosuppressants. Figure 1 shows the distribution of patients� drug therapy by pharmacological group. The most chosen AS in the �1 corticosteroid� group was topical clobetasol propionate (n=30;38.0%), followed by betamethasone dipropionate (n=17;21.5%). Looking at the �1 corticosteroid + 1 antihistamine� group, the most common combination was clobetasol propionate with ebastine (n=20;44.4%). When �2 corticosteroids� were used, the combination was never two systemic drugs, and prednisolone was always the systemic AS chosen to combine with a topical one.
�
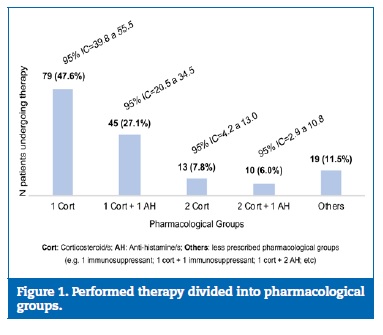
�
Immunosuppressants only represented 3.0% of the prescriptions (n=5), being the tacrolimus 0.1% ointment the most common (n=3). Corticosteroids were the most prescribed drugs, making up a total of 187 prescriptions, alone or in a combination. Table 4 reveals the corticosteroids� distribution by AS. Concerning their route of administration, the most widely used was the topical one (n=148;79.1%), where the application was made on the skin (n=138), on the oral mucosa (n=7), or intralesionally (n=3). The oral route was chosen in 24 of the 25 systemic administrations. The most common posology in pills was prednisolone 20mg/day (n=12;52.2%), followed by 30mg/day (n=4) and 40mg/day (n=4). Follow‑up was registered in 144 of the 166 patients who did drug therapy (86.7%). Figure 2 shows the result of different therapeutic regimens in the remission of LP lesions and their symptomatology. The ASs most associated with a positive result (cure/improvement) were clobetasol proprionate (n=14), betamethasone dipropionate (n=11), and the combination clobetasol proprionate with ebastine (n=15).
�
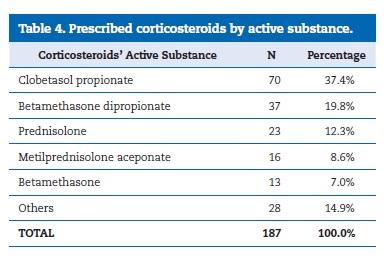
�
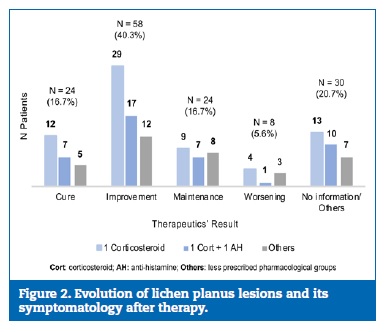
�
The disease�s evolution was variable, with an average time of expression close to 6.5 months for the population cured after the first appointment. There were 21 cases of disease recurrence (12%), where the mean time without symptoms/ lesions until a new exacerbation was 513 days. This period was not influenced by the therapeutic regimen.
Discussion
Although some studies state that LP affects more females than males,4, 20, 23 others agree that there is no gender trend for this pathology.6, 8, 24, 26 This study agreed with the latter, with no statistical difference between genders (p=0.820). Regarding age distribution, the first manifestation of LP was most common in the patients� 4th and 5th decades of life, followed by the 3rd and 6th. This result, as well as the fact that the youngest patient was 11 years old, is in agreement with other studies.4, 8, 20, 23, 25, 26
In Bhattacharya et al.�s publication, males presented the peak of LP lesions two decades earlier than females, for whom the peak occurred between the ages of 40 and 50.25 Usatine et al. explained that lesions occur more frequently in women during their perimenopause period.27 However, this investigation�s results are not in agreement with those authors since there was no statistical difference in the age distribution between genders (p=0.989).
The distribution of LP lesions in this study reveals a higher skin involvement and a lower oral and/or genital mucous involvement compared to results in the literature.11, 24, 25 Namely, the percentage of genital lesions found (10.3%) is similar to some published articles,24, 25 but lower than others.4 - 6
Regarding the topographic distribution of skin lesions, some epidemiological studies showed results similar to ours, with slight variations.24, 26 The literature also reports a greater amount of lesions in the upper and lower limbs� flexor zones, wrists, and ankles.6. 8. 24. 25. 27
In this study, the oral cavity was only affected in 17.2% of the cases, whereas other epidemiological studies reported higher values, between 20.6% and 42%.11, 24 ‑ 26 Nevertheless, all these values are below those documented by the American Academy of Oral and Maxillofacial Pathology in 2016, which stated that, apart from patients who have OLP as the single manifestation of the disease, about 60% of individuals with CLP presented oral cavity lesions.4 The low percentage of OLP lesions in this study may be explained by it being conducted in a Dermatology Service, where patients invariably have more skin lesions. Moreover, since the most prevalent subtype of OLP is asymptomatic, patients might not notice their pathology and not look for a healthcare professional.
The higher incidence of oral lesions in the buccal mucosa is supported by the literature.5, 6, 23 ‑ 25 Moreover, there were no cases of malignant transformation of OLP, maybe due to the reduced sample of oral lesions. Recent studies reveal the evolution of OLP lesions to squamous cell carcinoma in 1.09%-1.37% of patients.12, 13, 28
Regarding therapeutics, the most prescribed drugs were corticosteroids, either alone or in a therapeutic scheme. In fact, corticosteroids constitute the first‑line treatment for LP.5 8 29 - 31
However, some authors alert to the need for further studies evaluating the effectiveness of corticotherapy, especially the systemic one, compared to other treatment options.2, 19, 22, 32 - 34
The topical route of administration was the most widely used to apply corticotherapy, which is in agreement with the literature.5 19 30 - 33 Clobetasol propionate was the most prescribed active substance (37.4%), as reported in the literature.2 19 29 31 - 35
Prednisolone was the most commonly prescribed drug for systemic administration of corticosteroids (12.3%), generally in a dose of 20mg/day and along with a topical corticosteroid. Patil et al. and Tziotzios et al. also reported prednisolone as the most frequent corticosteroid, but with a dosage of 40-80mg/day and 30-60mg/day, respectively.29, 36
Antihistamines were the second most prescribed group in this study. Although their use is controversial,27. 37 their purpose is to reduce itching, the most common symptom in CLP(37). Immunosuppressants were prescribed much less frequently.
However, some recent articles support the use of tacrolimus 0.1% ointment instead of a topical corticosteroid.30, 31
Regarding the patients� follow-up, total remission of symptoms and resolution of LP lesions (or evolution to residual post-inflammatory hyperpigmentation) were the criteria considered as indicators of cure of the disease. The words �cure� and �recurrence� are used within the article to ease the reading, as these terms always refer to events of remission and exacerbation of symptoms and lesions, respectively, since LP as an autoimmune disease has no cure.6, 19 Despite the results reflecting that �1 corticosteroid� and �1 corticosteroid + 1 antihistamine� were the pharmacological groups more associated with situations of cure or improvement, it is not possible to conclude that these drugs are better because they are administered more often than the others.
No seasonal variation in LP manifestations was observed (p=0.235), similar to Bhattacharya et al.�s epidemiological study.25 Patients who healed after the first appointment took about 6.5 months to cure, similar to 49.1% of patients in that study.25 Regarding the disease�s recurrence, that epidemiological study also revealed a similar rate of relapses (10.3%).25
This study has some limitations, including incomplete medical records in some cases, which led to information loss since those patients were excluded. Moreover, this investigation�s results cannot be extrapolated to the general population as these patients were observed and medicated by the same group of specialists, which represents a bias.
Conclusions
In this research, LP affected both genders similarly and manifested itself mainly in adulthood. Its lesions occurred mostly in the skin, involving more the trunk, limbs, and extremities.
In a smaller portion, oral and genital mucosa were also affected. In the oral cavity, most lesions were observed in the buccal mucosa. The most prescribed drugs for LP management were corticosteroids, followed by antihistamines and immunosuppressants. Within corticotherapy, topical drugs were the most common ones, namely clobetasol propionate and betamethasone dipropionate. The most prescribed systemic corticosteroid was prednisolone.
Antihistamines were all administered orally, and ebastine was the most frequent. It was not possible to conclude which therapeutic regimen is more effective. The mean time of the disease varied largely, approximately 6.5 months. For the population with recurrence, the mean time without symptoms was 513 days.
�
Ethical disclosures
Protection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of data. The authors declare that they have followed their work center protocols on access to patient data and for its publication.
Right to privacy and informed consent. The authors declare that no patient data appear in this article.
�
Conflict of interest. The authors have no conflicts of interest to declare.
�
Acknowledgements. Research Centre of Statistical and Applications of University of Lisbon (CEAUL) under the project UIDB/00006/2020, Funda��o Nacional para a Ci�ncia e a Tecnologia (FCT), Portugal.
�
* The active substance and the corresponding a) to e) data were only collected if there was any AS from that pharmacological group. If there were more than one AS from the same group, all were collected together with each one�s corresponding a) to e) data.
�
References
1. Suresh SS, Chokshi K, Desai S, Malu R, Chokshi A. Medical management of oral lichen planus: A systematic review. J Clin Diagn Res. 2016;10:10-5.
2. Davari P, Hsiao HH, Fazel N. Mucosal lichen planus: An evidence-based treatment update. Am J Clin Dermatol. 2014;15:181-95.
3. Garcia-Pola MJ, Gonzalez-Alvarez L, Garcia‑Martin JM. Tratamiento del liquen plano oral. Revision sistem�tica y protocolo de actuacion. Med Clin. 2017;149:351-62.
4. Cheng YL, Gould A, Kurago Z, Fantasia J, Muller S. Diagnosis of oral lichen planus: a position paper of the American Academy of Oral and Maxillofacial Pathology. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122:332-54.
5. Alrashdan MS, Cirillo N, McCullough M. Oral lichen planus: a literature review and update. Arch Dermatol Res. 2016;308:539-51.
6. Gorouhi F, Davari P, Fazel N. Cutaneous and mucosal lichen planus: a comprehensive review of clinical subtypes, risk factors, diagnosis, and prognosis. Sci World J. 2014;2014:742826.
7. Ryan K, Hegarty AM, Hodgson T. Aetiology, diagnosis and treatment of oral lichen planus. Br J Hosp Med. 2014;75:492-6.
8. Weston G, Payette M. Update on lichen planus and its clinical variants. Int J Womens Dermatol. 2015;1:140-9.
9. Manousaridis I, Manousaridis K, Peitsch WK, Schneider SW. Individualizing treatment and choice of medication in lichen planus: a step by step approach. J Dtsch Dermatol Ges. 2013;11:981-91.
10. Olson MA, Rogers RS, Bruce AJ. Oral Lichen Planus. Clin Dermatol. 2016;34:495-504.
11. Ireddy SG, Udbalkar SG. Study of Lichen Planus and its different types and associated conditions. BMR Med. 2014;1:1-11.
12. Giuliani M, Troiano G, Cordaro M, Corsalini M, Gioco G, Lo Muzio L, et al. Rate of malignant transformation of oral lichen planus: A systematic review. Oral Dis. 2019;25:693-709.
13. Aghbari SMH, Abushouk AI, Attia A, Elmaraezy A, Menshawhy A, Ahmed MS, et al. Malignant transformation of oral lichen planus and oral lichenoid lesions: A metaanalysis of 20095 patient data. Oral Oncol. 2017;68:92-102.
14. Navas-Alfaro SE, Fonseca EC, Guzman-Silva MA, Rochael MC. Analise histopatologica comparativa entre liquen plano oral e cutaneo. J Bras Patol Med Lab. 2003;39:351-60.
15. Kurago ZB. Etiology and pathogenesis of oral lichen planus: An overview. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122:72-80.
16. Mester A, Lucaciu O, Ciobanu L, Apostu D, Ilea A, Campian RS. Clinical features and management of oral lichen planus (OLP) with emphasis on the management of hepatitis c virus (HCV)-related OLP. Bosn J Basic Med Sci. 2018;18:217-23.
17. Ma J, Zhang J, Zhang Y, Lv T, Liu J. The magnitude of the association between human papillomavirus and oral lichen planus: A meta-analysis. PLoS One. 2016;11:1-12.
18. Cerqueira JDM, Moura JR, Arsati F, Lima-Arsati YBO, Bittencourt RA, Freitas VS. Psychological disorders and oral lichen planus: A systematic review. J Investig Clin Dent. 2018;9:e12363.
19. Gonzalez‑Moles MA, Bravo M, Gonzalez-Ruiz L, Ramos P, Gil-Montoya JA. Outcomes of oral lichen planus and oral lichenoid lesions treated with topical corticosteroid. Oral Dis. 2018;24:573-9.
20. Le Cleach L, Chosidow O. Lichen Planus: Clinical Practice. N Engl J Med. 2012;366:723-32.
21. Das A, Panda S. Use of Topical Corticosteroids in Dermatology: An Evidence‑based Approach. Indian J Dermatol. 2017;62:237-50.
22. Atzmony L, Reiter O, Hodak E, Gdalevich M, Mimouni D. Treatments for Cutaneous Lichen Planus: A Systematic Review and Meta‑ Analysis. Am J Clin Dermatol. 2016;17:11-22.
23. Varghese SS, George GB, Sarojini SB, Vinod S, Mathew P, Mathew DG, et al. Epidemiology of Oral Lichen Planus in a Cohort of South Indian Population: A Retrospective Study. J Cancer Prev. 2016;21:55-9.
24. Tickoo U, Bubna AK, Subramanyam S, Veeraraghavan M, Rangarajan S, Sankarasubramanian A. A clinicopathologic study of lichen planus at a tertiary health care centre in south India. Pigment Int. 2016;3:96‑101.
25. Bhattacharya M, Kaur I, Kumar B. Lichen planus: A clinical and epidemiological study. J Dermatol. 2000;27:576-82.
26. Nasreen S, Ahmed I, Wahid Z. Associations of lichen planus: A study of 63 cases. J Pakistan Assoc Dermatologists 2007;17:17-20.
27. Usatine RP, Tinitigan M. Diagnosis and treatment of lichen planus. Am Fam Physician. 2011;84:53-60.
28. Fitzpatrick SG, Hirsch SA, Gordon SC. The malignant transformation of oral lichen planus and oral lichenoid lesions: A systematic review. J Am Dent Assoc. 2014;145:45�56.
29. Patil S, Khandelwal S, Sinha N, Kaswan S, Rahman F, Tipu S. Treatment modalities of oral lichen planus: an update. J Oral Diag. 2016;1:47-52.
30. Chamani G, Rad M, Zarei MR, Lotfi S, Sadeghi M, Ahmadi Z. Efficacy of tacrolimus and clobetasol in the treatment of oral lichen planus: A systematic review and meta‑analysis. Int J Dermatol. 2015;54:996-1004.
31. Hettiarachchi PVKS, Hettiarachchi RM, Jayasinghe RD, Sitheeque M. Comparison of topical tacrolimus and clobetasol in the management of symptomatic oral lichen planus: A double-blinded, randomized clinical trial in Sri Lanka. J Investig Clin Dent. 2017;8:1-6.
32. Lodi G, Carrozzo M, Furness S, Thongprasom K. Interventions for treating oral lichen planus: A systematic review. Br J Dermatol. 2012;166:938-47.
33. Husein‐ElAhmed H, Gieler U, Steinhoff M. Lichen planus: a comprehensive evidence‐based analysis of medical treatment. J Eur Acad Dermatol Venereol. 2019;33:1847-62.
34. Fazel N. Cutaneous lichen planus: A systematic review of treatments. J Dermatolog Treat. 2015;26:280-3.
35. Bagan J, Compilato D, Paderni C, Campisi G, Panzarella V, Picciotti M, et al. Topical therapies for oral lichen planus management and their efficacy: A narrative review. Curr Pharm Des. 2012;18:5470-80.
36. Tziotzios C, Brier T, Lee JYW, Saito R, Hsu CK, Bhargava K, et al. Lichen planus and lichenoid dermatoses: Conventional and emerging therapeutic strategies. J Am Acad Dermatol. 2018;79:807-18.
37. Welz‑Kubiak K, Reich A, Szepietowski JC. Clinical aspects of itch in lichen planus. Acta Derm Venereol. 2017;97:505-8.
�
Mariana Rodrigues Rebelo
E-mail address: rebelo.mariana@campus.ul.pt
