
Revista Portuguesa de Estomatologia, Medicina Dentária e Cirurgia Maxilofacial
Revista Portuguesa de Estomatologia Medicina Dentária e Cirurgia Maxilofacial | 2020 | 61 (2) | 45-51
Original research
Effect of contingent electrical stimulation in sleep bruxism – a randomized clinical trial
Efeito da estimulação elétrica funcional no bruxismo do sono – estudo clínico randomizado
a Faculdade Medicina Dentária, Universidade de Lisboa, Lisbon, Portugal
Maria Carlos Quaresma - mcrealdias@hotmail.com
Article Info
Rev Port Estomatol Med Dent Cir Maxilofac
Volume - 61
Issue - 2
Original research
Pages - 45-51
Go to Volume
Article History
Received on 06/04/2020
Accepted on 13/06/2020
Available Online on 10/09/2020
Keywords
Original research
�
Effect of contingent electrical stimulation in sleep bruxism � a randomized clinical trial
Efeito da estimulacao eletrica funcional no bruxismo do sono � estudo clinico randomizado
�
Maria Carlos Quaresma*, Pedro Crispim, Henrique Luis, Duarte Marques, Jo�o Caram�s
Faculdade Medicina Dent�ria, Universidade de Lisboa, Lisbon, Portugal
�
�
http://doi.org/10.24873/j.rpemd.2020.09.705
�
ABSTRACT
Objectives: A randomized clinical trial comparing the effects of a 4- and 8-week consecutive use of contingent electrical stimulation on self-reported pain, jaw muscle activity, and threshold intensity in teeth grinding during sleep, over 6 months.
Methods: This randomized clinical trial studied 48 patients diagnosed with bruxism and masticatory myofascial pain according to established methods. Patients were randomly allocated to Group I � 4-week contingent electrical stimulation (n=24), and Group II � 8-week contingent electrical stimulation (n=24). The primary outcomes assessed were the number of electromyographic events per hour of sleep, numerical rating scale pain scores, and threshold intensity when grinding. Analysis of variance models was used to compare results at a 5% significance level.
Results: There was a statistically significant (p<0.05) decrease in pain level (-71.7% and -71.2%, respectively) and grinding mean intensity (-37.4% and -44.9%, respectively) at the 6-month follow-up for Group I. Contingent electrical stimulation reduced pain and the threshold intensity of grinding at night in patients with masticatory myofascial pain with definitive sleep bruxism, with a positive correlation (p<0.05) between the two primary outcomes.
Conclusions: The results of this study suggest that both the 4- and 8-week contingent electrical stimulation protocols are effective in reducing patient symptoms related to sleep bruxism.
Keywords: Biofeedback, �Conditioning electrical stimulation, Grinding, Pain, Sleep bruxism,< Temporal muscle
�
RESUMO
Objetivos: Ensaio cl�nico randomizado para compara��o dos efeitos de 4 e 8 semanas do uso consecutivo de estimula��o el�trica funcional na dor autorreferida, atividade muscular da mand�bula e intensidade m�dia no ranger de dentes durante o sono por mais de 6 meses.
M�todos: Este ensaio cl�nico randomizado estudou 48 pacientes diagnosticados de acordo com m�todos estabelecidos, com bruxismo do sono e dor miofascial. Os pacientes foram alocados aleatoriamente no grupo I � estimula��o el�trica funcional de 4 semanas (n=24) e no grupo II � 8 semanas de estimula��o el�trica funcional (n=24). Os par�metros prim�rios avaliados foram o n�mero de epis�dios presentes na eletromiografia por hora de sono, valores de dor na escala num�rica de dor e intensidade m�dia de rangido por noite. Foram utilizados modelos de an�lise de vari�ncia para comparar os resultados, estabelecendo-se um n�vel de signific�ncia de 5%.
Resultados: Houve redu��o estatisticamente significante (p<0,05) no n�vel de dor (-71,7% e -71,2%, respetivamente) e intensidade m�dia de rangido (-37,4% e -44,9%, respetivamente) no seguimento de 6 meses para o grupo I. A estimula��o el�trica funcional reduziu a dor e a intensidade m�dia de rangido por noite em pacientes com dor miofascial e bruxismo do sono (diagn�stico definitivo), com uma correla��o positiva (p<0,05) entre os dois par�metros prim�rios.
Conclus�es: Os resultados deste estudo sugerem que a estimula��o el�trica funcional em protocolos de 4 e 8 semanas s�o eficazes na redu��o dos sintomas dos pacientes relacionados ao bruxismo do sono.
Palavras-chave: Biofeedback, Estimula��o el�trica funcional, Rangido, Dor, Bruxismo do sono, M�sculo temporal
�
Introduction
Bruxism, in general, is a repetitive muscle condition characterized by the grinding and/or clenching of the teeth when awake or asleep.1 International consensus on its definition gave rise to several questions, as sleep and awake bruxism are generally considered different behaviors observed during sleep and wakefulness, respectively.2 Sleep bruxism (SB) is defined as a repetitive jaw muscle activity characterized by clenching or grinding of the teeth or bracing or thrusting of the mandible during sleep.3 Even though, according to the current view, bruxism is regulated mainly centrally, and not peripherally (i.e., not caused by anatomical factors like certain characteristics of dental occlusion and articulation), its etiology is still controversial.2 Clinical and scientific evidence suggest that bruxism could be related to periods of physical and emotional stress and the anticipation of such periods,4 with a multifactorial pattern being the most plausible hypothesis, in which psychosocial and pathomorphological factors interact with peripheral morphological factors.4, 5
SB can generate high occlusal forces, which are sustained by the teeth, supporting tissues, and temporomandibular joint.
It can cause attrition, tooth wear and fracture, hypersensitivity, periodontal ligament lesions, pulpitis and tooth necrosis, fatigue and muscle pain, buccal movement limitations, temporal region headaches, and temporomandibular disorders.6 ‑ 8
The diagnosis of SB can be based on reported tooth grinding or clenching accompanied by one of the following three signs: abnormal dental wear, sounds associated with bruxism, and muscle discomfort, fatigue or stiffness when awaking.9, 10 Self‑reporting, together with clinical examination methods, can define a �probable� SB diagnosis, but the definite diagnosis should be based on electrophysiological monitoring undertaken in sleep clinics or with a portable device.
One of the therapeutic targets in a patient with bruxism is to modify or decrease the parafunctional activity. Several techniques have been used to control it, namely, hypnosis, occlusal adjustment, muscle relaxant splints, physiotherapy and muscle‑relaxing exercises, medication, acupuncture, and biofeedback,6, 11 ‑ 13 but, to date, there is little evidence on the effectiveness of the different treatments.14 More recently, a therapeutic alternative based on a contingent electrical stimulation (CES) mechanism has achieved promising results.14, 15 It consists of a signal that is sent to the temporal or masseter muscle, inducing immediate relaxation.16 The literature suggests that biofeedback devices could be linked to the relaxation reflex (or exteroceptive suppression period), 14, 17, 18 thus presenting an alternative to conventional treatments for the remission and control of parafunctional activity.8, 12, 19, 20 Several authors have reported it as an effective method for a faster alleviation of chronic headache symptoms, myofascial pain, and muscular inflammation when compared to other therapeutic alternatives.15, 21 However, the duration of treatment needed to obtain a measurable relaxation of the jaw muscles and effective stabilization of the symptoms is still unclear.
Over recent years, the industry has evolved toward producing more portable and friendlier CES devices. GrindCare (GrindCare, Medotech A/S, Denmark) consists of a biofeedback device capable of measuring electromyography (EMG) activity of the anterior temporalis muscle, by emitting a painless electrical pulse to the temporal region when EMG activity exceeds the individually determined threshold.14
The aim of this randomized clinical trial was to evaluate the effect of 4‑ and 8‑week treatment protocols with CES on pain symptoms, EMG activity (in the anterior temporalis muscle), and threshold intensity when grinding (per night) on SB patients with myofascial pain.
Material and Methods
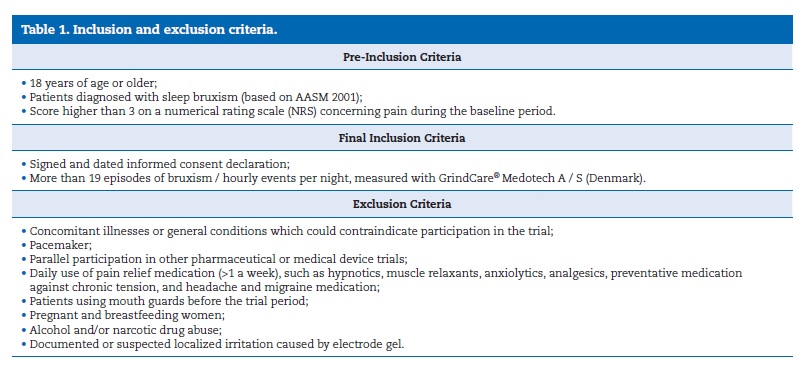
A single‑center, randomized, two‑arm, parallel clinical trial was performed. This study employed a sample of 48 volunteers recruited according to previously established inclusion and exclusion criteria (Table 1) at the clinic of the Faculty of Dental Medicine of the University of Lisbon. The ethical committee of this institution approved the study protocol, which was conducted in full compliance with the World Medical Association Declaration of Helsinki and its most recent amendments, following the approved clinical practice guidelines.
�
�
When accepted into the study, patients were randomly allocated to Group I or Group II by a computer‑generated randomization software (GraphPad Quick‑Calcs website: http://www.graphpad.com/quickcalcs/randomize1.cfm). The code for randomization was kept in a sealed envelope and opened only at the end of the study. Data were analyzed by a third party blinded to the allocation results, which were referred to as treatment I or II in the SPSS worksheet (SPSS, Inc., Chicago, IL, USA). The medical record was assessed by a single trained and calibrated researcher according to previously established methods (Dworkin and LeResche 1992 � RDC‑TMD 1992).
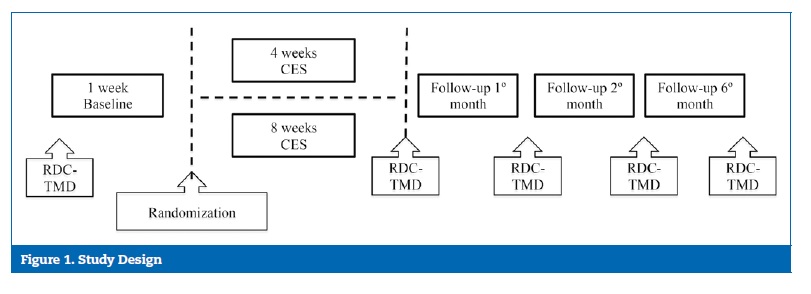
The study workflow is shown in Figure 1 (Group I � 4‑week CES and Group II � 8‑week CES). EMG of the anterior temporalis muscle was monitored daily over the first 8 weeks of the follow‑up period to evaluate the short‑term effect, and then at 6 months (7 consecutive nights) to evaluate the long‑term effect. Patients were instructed to complete a diary designed to assess sleep quality and symptomatology according to: i) level of pain (numerical rating scale [NRS] classification from 0‑10), ii) fatigue and muscle tension (NRS classification from 0‑10), iii) adverse events. The clinician calibrated each EMG device individually on day 0, and each participant adjusted stimulus intensity for CES daily.
�
�
The EMG device uses a signal recognition algorithm14 based on the signals collected from three electrode contacts.
An EMG episode is recorded when the amplitude of the EMG signal exceeds the pre‑ defined threshold of more than 100 ms for up to 1s. Longer‑lasting EMG events are counted as additional events.22 The EMG activity was expressed as the number of grinds per hour of sleep (NG), by previously established methods. Briefly every night, participants were requested to relax their jaw muscles for 10 s and then clench their teeth at approximately 60% of the maximum voluntary contraction for 10s (verbal explanation, with clinical counseling and visual training). The number of events was determined based on the algorithm previously established, with events defined as EMG activity higher than the signal level at rest plus 20% of the maximum EMG level during the 60% contraction.14
Pain level was scored as 0 for no pain and 10 for the highest pain experienced. Contraction intensity was expressed as a threshold intensity (TI) number per night of sleep.
This study was conducted as a pragmatic randomized clinical trial to determine the effectiveness of these interventions in a real‑world setting. Although the two treatment protocols differed, the measurements of interest (EMG episodes and activity) were objective and not susceptible to interpretation, thus removing the potential bias for lack of blinding.
Kolmogorov‑Smirnov normality tests were performed for the study variables. Only the TI variable presented a non‑normal distribution (p<0.001). Whenever data were analyzed with this variable, non‑parametric tests were used. The EMG/h, pain score, and TI data were described. Baseline (1st week � Bas) data was used to evaluate the correlation between pain score and EMG activity or TI parameters for the entire sample using the appropriate tests (Pearson�s coefficient or Spearman�s rho tests). Multiple comparisons between variable means were performed using a t‑test with the Bonferroni correction. The level of significance was set at 5%.
Results
A total of 48 patients were randomly allocated to one of the groups, and there were no dropouts throughout the study.
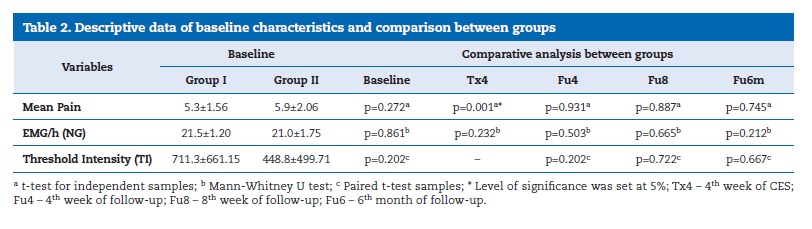
The baseline characteristics of the two groups are shown in Table 2. Student�s t‑test was employed to test differences between baseline characteristics in both groups with significant differences for TI.
�
�
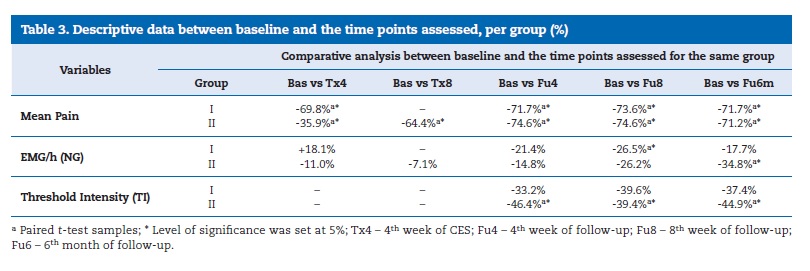
Statistically significant differences were observed in mean pain levels between the two groups after one month of treatment (end of active treatment for Group I and half of active treatment for Group II) and at each time point of the follow‑up period for both groups (Table 3).
�
�
In Group I, NG was 21.5 �} 1.20 at Bas, 25.5 �} 28.54 at Tx4 (4th week of CES), 16.9 �} 10.88 at Fu4 (4th week of follow‑up), 15.8 �} 11.6 at Fu8 (8th week of follow‑up), and 17.7 �} 13.31 at Fu6m (6th month of follow‑up), with statistically significant differences between Bas and Fu8 (paired samples, t‑test, p<0.05). In
Group II, NG was 21.0 �} 1.75 at Bas, 18.7 �} 16.32 at Tx4, 19.5 �} 17.74 in Tx8, 17.9 �} 17.69 at Fu4, 15.5 �} 15.91 at Fu8, and 13.7 �} 10.63 at Fu6m, with statistically significant differences between Bas and Fu6 and between Tx8 and Fu6m (paired samples, t‑test, p<0.05). No statistically significant differences (p>0.01; Mann‑Whitney U test) were found for NG between the two groups in the assessed time points (Table 3).
TI in Group I was 711.3 �} 661.15 at Bas, 475.1 �} 487.02 at Fu4, 429.8 �} 501.16 at Fu8, and 445.6 �} 518.84 at Fu6m. When comparing the baseline appointment with the first three controls (Fu4, Fu8, Fu6m), statistically significant differences (paired t‑ test samples) were verified, with p=0.004, p=0.001, and p=0.04, respectively. The mean follow‑up was not statistically significant.
The TI level of Group II was 448.8 �} 499.71 at Bas, 240.7 �} 311.45 at Fu4, 272.0 �} 284.47 at Fu8, and 247.4 �} 176.84 at Fu6m.
In a comparison (using paired t‑test samples) between Bas and the first, second, and third controls (Fu4, Fu8, and Fu6m), statistically significant differences (p<0.001) were observed, with decreasing values for the TI level. When comparing the mean TI between both groups (I and II), no statistically significant differences were found in the different time points. The comparative analysis of the different pair variables in each group was performed using the Spearman�s rho test for the same time points and the Pearson�s test for Fu8 in Group I regarding the pair Mean Pain‑TI.
In Group I, at baseline, the Mean Pain‑NG pair showed a moderate positive correlation (ρ = 0.415), with statistical significance (p=0.044). In Tx1, Tx2, Tx3, and Tx4, it had a moderate to very weak correlation (ρ = 0.545, ρ = 0.370, ρ = 0.120 and ρ = 0.125), with a non‑significant p value at every stage except Tx1 (p=0.006). In Fu4 and Fu8, a moderate Mean Pain‑NG correlation was observed (ρ = 0.428 and ρ = 0.451, respectively), with statistical significance (p=0.037 and p=0.027, respectively). In Fu6m, this correlation (ρ = 0.366) had no statistical significance (p=0.108), becoming weaker over the course of the study. In Group II, a weak Mean Pain‑NG correlation was detected in Bas (ρ = 0.248; p = 0.242). In Tx1, Tx2, Tx3, Tx4, Tx5, Tx6, Tx7, and Tx8, that correlation was weak/very weak (ρ = 0.048, ρ = 0.060, ρ = 0.253, ρ = 0.253, ρ = 0.051, ρ = 0.202, ρ = 0.268 and ρ = 0.286, respectively), although without statistical significance (p>0.05) at every stage of the active treatment, except Tx1 (p=0.048). In the control period � Fu4, Fu8 and Fu6m, no correlation was detected (ρ = 0.184, ρ = ‑ 0.030, and ρ = ‑ 0.085, respectively) between NG and Mean Pain, with no statistical significance in all readings.
In Group I, at baseline, a moderate positive correlation (ρ = 0.423; p=0.116) was observed in the Mean Pain‑TI pair. In Fu4, there was a strong positive Mean Pain‑TI correlation (ρ = 0.781), with a p=0.001, showing that those who started to feel less pain, started to grind less intensely. The same occurred in Fu8 and Fu6m, where there were strong correlations (ρ = 0.792 and ρ = 0.835, respectively), with p<0.001 in both. In Group II, a very weak Mean Pain‑TI correlation (ρ = 0.116, ρ = 0.110, ρ = 0.144, and ρ = 0.142, respectively) was observed in all study weeks (Bas, Fu4, Fu8, and Fu6m); i.e., both variables decreased throughout the study, but with no statistical relevance.
In Group I, at baseline, the NG‑TI pair showed a strong positive correlation (ρ = 0.705; p=0.003), meaning that those who experienced the highest level of grinding did so with the greatest intensity. In Fu4, Fu8, and Fu6m, there was also a statistically significant moderate positive correlation between NG and TI (ρ = 0.582, ρ = 0.614 and ρ = 0.537, respectively). In Group II, a very weak/weak correlation (ρ = 0.046 and ρ = 0.375, respectively) was observed at Bas and Fu4, with no statistical significance in any of the cases. At two months under the control regime, this interaction became strong with statistical significance (ρ = 0.745 and p=0.001). However, at 6 months, it lost statistical significance and became a weak correlation (ρ = 0.221).
�
Discussion
Following the available literature and scientific evidence, the treatment protocol was proposed to assess how SB patients experiencing pain would respond to medium‑and long‑term treatments with a CES device. The use of CES reduced the pain and TI of grinding per night in patients with masticatory myofascial pain with definitive SB. EMG activity on the temporalis muscle had an inconsistent evolution after CES therapy.
Baseline characteristics of the study population were similar between the study groups, except for the TI, and pain relief was observed in both protocols, with no recurrence throughout the study. However, the minor improvements observed after the 4‑week treatment suggest that extended treatment protocols may offer no further advantages. Thus, a protocol with 1 month of CES appears to be sufficient. The authors suggest that, in a clinical setting, shorter protocols are more favorable and achieve the same results.
Since the inclusion criteria in this study were not based on gender, but rather on complaints and reasons for consultation, it was not possible to attribute a real relationship between prevalence and epidemiology of gender and SB.
A decrease in temporal muscle activity and concomitant myofascial pain in patients with SB had been previously reported,15 corroborating our findings. Another study,23 with a design comparable to the present one, with 19 individuals diagnosed with SB (using the AASM criteria),24 observed a 56.9% pain relief in 58% of the sample � the motivated group, and a 28.8% pain relief in 42% of the sample � the group considered skeptical in the initial analysis. The motivated group values were similar to those found in the present study (pain relief in 69.8% and 64.4% in Groups I and II, respectively). In light of the results, the authors hypothesized the importance of the parameter �duration of the bruxism event� and its �intensity� in the perpetuation of pain, supporting the hypothesis that long and short events are signaled equally by the CES device, regardless of their intensity. A systematic review published in 2014, pointed out that CES had the potential to induce long‑term changes in behaviors that could include the reduction or elimination of patient symptoms. 25 A systematic review published in 2015 highlighted that it was yet to be proven that a reduction in muscle activity could help decrease the patient�s clinical pain.26 The present study corroborated these statements, emphasizing the importance of diagnostic consultation for the adoption of adequate and effective therapeutic strategies.
As a general observation, it is important to note that none of the participants reported complaints concerning sleep throughout the study, similar to the results obtained by a prerevviously published study.14 Also, the GrindCareR system worked well as a convenient portable device inducing a stimulus on a unilateral temporal muscle.
As there was no consistent evolution in the grinding activity in both protocols (Group I or II), pain might not be related to the number of grind events, as mentioned by other authors,27 who pointed out that, in this clinical situation, care must be taken to not create cause‑effect relationships that are too simplistic. This clinical trial showed that, during the treatment time (in both groups), the correlation between pain and muscular activity disappeared, evidencing the efficacy of CES.
Also, at 6 months post‑ treatment, there was no correlation between muscle activity and pain in both groups.
The inconsistent outcome for NG following CES therapy is moderately in line with the literature, with results showing a decrease in EMG of 35%�56.9% in the active treatment period and 31�38% in the control period.14, 15, 22, 28 ‑ 31 Our findings were smaller, but with a higher sample size, which implies decreased intrinsic variability and increased statistical power of the results obtained. In fact, the present study highlights the fact that, besides being inconsistent, the evolution of NG throughout the study was also not statistically significant. In a study published in 2013,23 whose design was similar to Group I, the authors detected an inconsistent development of NG with a wide range in NG parameters and consequent response to CES. The present study corroborates this finding. Although a recent study22 found that EMG event frequency could return to baseline levels in the follow‑up, it also suggested the need for more studies on this subject with larger sample sizes and follow‑up periods.
Central neuronal alterations (central sensitization), typical of chronic conditions,32 can also explain the weak correlation between pain and EMG activity found in the present research.
The authors suggest that intensity is the main contributor to pain recorded by the patient.
Regarding the TI, the proposed treatments were effective, with a statistically significant decrease during the active treatment and in the control period. There was no recurrence of TI in values similar to the Bas value. Our results suggest that, clinically, shorter protocols are more prone to patient compliance, achieving the same results as longer protocols. The present work is in line with the literature23 and emphasizes the possible relationship between intensity and response to different treatments, as well as the importance of the wide range of individual responses that this variable elicits. The correlations between TI and pain, particularly in Group I, showed that the two variables were related from the beginning and emphasized the relationship between the treatment with CES and the controls.
As for limitations, the authors consider that, because of the study flowchart in phases and long control periods, calibration could be different in the baseline week and the 23rd week. Additionally, and although the study was conducted in the usual patient environment, there could be an over‑evaluation of the results, since the use of cables and devices on the patients could induce situations of bruxism. Despite the limitations of this study, CES reduced the pain and TI of grinding per night in patients with masticatory myofascial pain with definitive SB.
Conclusions
Both the proposed protocols presented clinical significance by leading to a reduction in pain and consequent increase in the patient‑ related outcomes. This decrease in the NRS pain score may have been influenced by the decreased grinding intensity.
Results suggest that a 4‑week protocol could be sufficient to relieve pain in SB. More studies on the subject of pain, with simple designs and effective solutions, for the assessment of these new devices should be performed.
�
References
1. Lobbezoo F, Ahlberg J, Glaros AG, Kato T, Koyano K, Lavigne GJ, et al. Bruxism defined and graded: an international consensus. J Oral Rehabil. 2013;40:2‑4.
2. Lobbezoo F, Ahlberg J, Raphael KG, Wetselaar P, Glaros AG, Kato T, et al. International consensus on the assessment of bruxism: Report of a work in progress. J Oral Rehabil. 2018;45:1-8.
3. Manfredini D, Serra‑Negra J, Carboncini F, Lobbezoo F. Current concepts of bruxism. Int J Prosthodont. 2017;30:437‑8.
4. Lobbezoo F, van der Zaag J, van Selms MK, Hamburger HL, Naeije M. Principles for the management of bruxism. J Oral Rehabil. 2008;35:509‑23.
5. Lobbezoo F, Visscher CM, Ahlberg J, Manfredini D. Bruxism and genetics: a review of the literature. J Oral Rehabil.2014;41:709‑14.
6. Attanasio R. Nocturnal bruxism and its clinical management. Dent Clin North Am. 1991;35:245‑52. PMID: 1997355
7. Nissani M. A bibliographical survey of bruxism with special emphasis on non‑traditional treatment modalities. J Oral Sci. 2001;43:73-83.
8. Jadidi F, Castrillon E, Svensson P. Effect of conditioning electrical stimuli on temporalis EMG activity during sleep. J Oral Rehabil 2008;35:171‑83.
9. American Academy of Sleep Medicine. International Classification of Sleep Disorders, Revised: Diagnostic and Coding Manual. Chicago, IL, America Academy of Sleep Medicine, 2001.
10. Carra MC, Huynh N, Lavigne GJ. Diagnostic accuracy of sleep bruxism scoring in absence of audio‑video recording: a pilot study. Sleep Breath. 2015;19:183‑90.
11. Wieselmann‑Penkner K, Janda M, Lorenzoni M, Polansky R. A comparison of the muscular relaxation effect of TENS and EMG‑biofeedback in patients with bruxism. J Oral Rehabil. 2001;28:849-53.
12. Nishigawa K, Kondo K, Takeuchi H, Clark GT. Contingent electrical lip stimulation for sleep bruxism: a pilot study. J Prosthet Dent. 2003;89:412-7.
13. Ommerborn MA, Schneider C, Giraki M, Schafer R, Handschel J, Franz M et al. Effects of an occlusal splint compared with cognitive‑behavioral treatment on sleep bruxism activity. Eur J Oral Sci. 2007;115:7-14.
14. Jadidi F, Castrillon E, Svensson P. Effect of conditioning electrical stimuli on temporalis electromyographic activity during sleep. J Oral Rehabil. 2008; 35:171‑ 83.
15. Bernhardt O, Hawali S, Sumnig W, Meyer G. Electrical stimulation of the temporalis muscle during sleep in case of myofascial pain � a pilot study. Journal of Craniomandibular Function 2012;4:197‑210.
16. Jadidi F, Wang K, Arendt‑nielsen L, Svensson P. Effects of different stimulus locations on inhibitory responses in human jaw‑closing muscles. J Oral Rehabil. 2011;38:487‑500.
17. Dubner R, Sessle BJ, Storey AT. Pain. In: The neural basis of oral and facial function. 1st edition. New York, Plenum Press, 1978. p. 9‑55.
18. Svensson P, Graven‑Nielsen T. Craniofacial muscle pain: Review of mechanisms and clinical manifestations. J Orofac Pain 2001;15:117‑45. PMID: 11443825
19. Rugh JD, Solberg WK. Electromyographic studies of bruxist behavior before and during treatment. J Calif Dent Assoc.1975;3:56-9. PMID: 1073399
20. Pierce CJ, Gale EN. A comparison of different treatments for nocturnal bruxism. J Dent Res.1988;67:597‑601.
21. Hudzinski LG, Walters PJ. Use of a portable electromyogram integrator and biofeedback unit in the treatment of chronic nocturnal bruxism. J Prosthet Dent. 1987;58:698‑701.
22. Raphael KG, Janal MN, Sirois DA, Dubrovsky B, Wigren PE, Klausner JJ et al. Masticatory muscle sleep background electromyographic activity is elevated in myofascial temporomandibular disorder patients. J Oral Rehabil. 2013;40:883‑91.
23. Needham R., Davies SJ. Use of the GrindCare device in the management of nocturnal bruxism: a pilot study. Br Dent J. 2013;215:E1.
24. American Academy of Sleep Medicine. International Classification of Sleep Disorders: Diagnostic and Coding Manual, 2nd edition Westchester, IL: American Academy of Sleep Medicine; 2005.
25. Ilovar S, Zolger D, Castrillon E, Car J, Huckvale K. Biofeedback for treatment of awake and sleep bruxism in adults: systematic review protocol. Syst Rev. 2014 3:42.
26. Manfredini D, Ahlberg J, Winocur E, Lobbezoo F. Management of sleep bruxism in adults: a qualitative systematic literature review. J Oral Rehabil. 2015;42:862‑74.
27. Svensson P, Jadidi F, Arima T, Baad‑Hansen L, Sessle BJ. Relationships between craniofacial pain and bruxism. J Oral Rehabil. 2008;35:524‑47.
28. Jadidi F, Castrillon E, Nielsen P, Baad‑Hansen L, Svensson P. Effect of contingent electrical stimulation on jaw muscle activity during sleep: a pilot study with a randomized controlled trial design. Acta Odontol Scand. 2013;71:1050‑62.
29. Conti PC, Stuginski‑Barbosa J, Bonjardim LR, Soares S, Svensson P. Contingent electrical stimulation inhibits jaw muscle activity during sleep but not pain intensity or masticatory muscle pressure pain threshold in self‑ reported bruxers: a pilot study. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117:45‑52.
30. Sumiya M, Mizumori T, Kobayashi Y, Inano S, Yatani H. Suppression of sleep bruxism: effect of electrical stimulation of the masseter muscle triggered by heart rate elevation. Int J Prosthodont. 2014;27:80‑6.
31. Gu W, Yang J, Zhang F, Yin X, Wei X, Wang C. Efficacy of biofeedback therapy via a mini wireless device on sleep bruxism contrasted with occlusal splint: a pilot study. J Biomed Res. 2015;29:160‑8.
32. Maixner W, Diatchenko L, Dubner R, Fillingim RB, Greenspan JD, Knott C et al. Orofacial pain prospective evaluation and risk assessment study�the OPPERA study. J Pain. 2011;12(11 Suppl):T4‑11. e1‑2.
�
Maria Carlos Real Dias Quaresma
E-mail address: mcrealdias@hotmail.com
�
Ethical disclosures
Protection of human and animal subjects. The authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of data. The authors declare that no patient data appear in this article.
Right to privacy and informed consent. The authors declare that no patient data appear in this article.
�
Conflict of interest
The authors have no conflicts of interest to declare.
�
Article history:
Received 6 April 2020
Accepted 13 July 2020
Available online 11 September 2020
