
Revista Portuguesa de Estomatologia, Medicina Dentária e Cirurgia Maxilofacial
SPEMD | 2019 | 60 (4) | 163-168
Original research
Evaluation of the buccal mucosa of patients with acute lymphocytic leukemia: A case series study
Avaliação da mucosa bucal de pacientes com leucemia linfocítica aguda: Estudo de série de casos
a Faculdade de Odontologia. Universidade Federal do Amazonas, Manaus, AM, Brasil
b Programa de Pós-Graduação em Odontologia (PPGO). Universidade Federal do Amazonas, Manaus, AM, Brasil
c Centro de Apoio Multidisciplinar (CAM). Universidade Federal do Amazonas, Manaus, AM, Brasil
d Departamento de Patologia e Medicina Legal. Faculdade de Medicina. Universidade Federal do Amazonas, Manaus, AM, Brasil
Tatiana Nayara Libório-Kimura - tliborio@ufam.edu.br, tatiana.liborio@gmail.comtliborio@ufam.edu.br, tatiana.liborio@gmail.com
Article Info
Rev Port Estomatol Med Dent Cir Maxilofac
Volume - 60
Issue - 4
Original research
Pages - 163-168
Go to Volume
Article History
Received on 18/09/2019
Accepted on 06/12/2019
Available Online on 02/01/2020
Keywords
Original research
�
Evaluation of the buccal mucosa of patients with acute lymphocytic leukemia: A case series study
Avalia��o da mucosa bucal de pacientes com leucemia linfoc�tica aguda: Estudo de s�rie de casos
�
Juliana Maria Souza de Oliveiraa,b, Juliana Vianna Pereiraa,b, �velyn Costa Lirac, Cristina Maria Borborema dos Santosc, Gerson de Oliveira Paiva Netob, Tatiana Nayara Lib�rio-Kimurab,d,*
a Faculdade de Odontologia. Universidade Federal do Amazonas, Manaus, AM, Brasil
b Programa de Pos-Graduacao em Odontologia (PPGO). Universidade Federal do Amazonas, Manaus, AM, Brasil
c Centro de Apoio Multidisciplinar (CAM). Universidade Federal do Amazonas, Manaus, AM, Brasil
d Departamento de Patologia e Medicina Legal. Faculdade de Medicina. Universidade Federal do Amazonas, Manaus, AM, Brasil
�
�
http://doi.org/10.24873/j.rpemd.2020.01.691
�
Abstract
Objectives: Prospective evaluation of the buccal mucosa of pediatric patients with acute lymphocytic leukemia (ALL) regarding its clinical aspect and expression of members from the HHV family.
Methods: From September 2014 to January 2015, a series of nine consecutive patients was evaluated, with ages ranging from 2 to 14 years, in treatment at the Amazon Hematology and Hemotherapy Foundation (HEMOAM). The buccal mucosa of those patients was clinically evaluated, screened for alterations and analyzed by PCR, to search DNA from HSV-1, CMV and EBV during four moments of the pre-phase/induction phase of the chemotherapy treatment (P/I) � D0/D1, D8, D15 and D35, as well as four moments of the consolidation of the remission phase (CR) � D1, D15, D29 and D50. The protocol proposed by the Brazilian Cooperative Group for Treatment of Childhood Lymphocytic Leukemia (GBTLI ALL-2009) was followed.
Results: A prevalence of 2-year-old children (66.7%, n=6) with a diagnose of B-cell ALL (88.9%, n=8) was observed. Buccal alterations were observed in 33.3% (n=3) of the patients: erythema (D35 P/I), dry lips (D8 and D15 P/I) and an episode of xerostomia (D15 P/I). None of the samples was positive for HSV-1 and CMV, but 33.3% (n=3) of the cases expressed EBV (D8 and D15 P/I).
Conclusions: Buccal alterations and the presence of HSV-1, CMV and EBV in patients with ALL was inexpressive, with most of the patients being sound throughout the treatment. Thus, it cannot be affirmed that the analyzed viruses are part of the microbiome of those patients. However, it has been suggested that the presence of EBV is more expected than HSV-1 and CMV.
Keywords: Acute lymphocytic leukemia, Herpesviridae,Oral mucositis,Polymerase Chain Reaction
�
Resumo
Objetivo: Avaliar prospectivamente a mucosa bucal de pacientes pedi�tricos com Leucemia Linfoblastica Aguda (LLA) quanto ao seu aspecto clinico e express�o de membros da fam�lia HHV.
M�todos: Roman;mso-bidi-font-family: CaeciliaLTStd-Roman'>No per�odo de setembro de 2014 a janeiro de 2015, foram avaliados consecutivamente, uma s�rie de nove pacientes com idades entre 2 e 14 anos atendidos na Funda��o de Hematologia e Hemoterapia do Amazonas (HEMOAM). A mucosa bucal desses pacientes foi avaliada clinicamente quanto a altera��es bucais e por meio de PCR para detec��o do DNA do HSV-1, CMV e EBV em 4 momentos da pr�-fase/indu��o (P/I), sendo eles D0/D1, D8, D15 e D35, assim como em 4 momentos da consolida��o da remiss�o (CR), sendo eles D1, D15, D29 e D50 pelo protocolo do Grupo Brasileiro de Leucemias na inf�ncia (GBTLI LLA 2009).
Resultados: Roman;mso-bidi-font-family: CaeciliaLTStd-Roman'>Foi observada maior preval�ncia de crian�as com 2 anos de idade (66,7%, n=6), do sexo masculino (66,7%, n=6) e com diagnostico de LLA de c�lulas B (88,9%, n=8). Altera��es bucais foram observadas somente em 33,3% (n=3) dos pacientes, sendo elas eritema (D35 P/I), l�bios ressecados (D8 e D15 P/I) e relato de xerostomia (D15 P/I). Nenhuma das amostras foi positiva para HSV-1 e CMV, no entanto 33,3% (n= 3) dos casos apresentaram express�o do EBV (D8 e D15 P/I).
Conclus�es: Roman;mso-bidi-font-family: CaeciliaLTStd-Roman'>Altera��es bucais e presen�a dos v�rus HSV-1, CMV e EBV nos pacientes com LLA avaliados mostrou-se inexpressiva, estando a maioria dos pacientes com mucosa h�gida ao longo do tratamento. N�o se pode afirmar que os v�rus analisados integrem a microbiota desses pacientes, no entanto, h� ind�cios de que a presen�a do EBV seja mais esperada que o HSV-1 e CMV.
Palavras-chave: Leucemia linfocitica aguda, Herpesviridae, Mucosite oral, Reac��o em cadeia da polimerase
�
Introduction
Acute lymphocytic leukemia (ALL) is the most common malignant neoplasm in childhood and adolescence with a prevalence peak at 2 to 5 years of age.1 - 3 It is frequently sensible to chemotherapy, having an event-free survival rate of 90% when the ideal therapeutic regimen, based on subgroup stratification, is adopted.4 This regimen�s treatment strategies comprehend phases, such as the induction, consolidation and maintenance phases.5
Secondary complications, such as mucocutaneous pallor, fever, asthenia, lymphadenopathy, xerostomia, cushingoid facies, hemorrhage, mucositis, candidiasis, ulcers and alterations on the saliva constituents with swallowing impairment may arise as a consequence of the chemotherapy treatment.6, 9 The presence of microorganisms is an aggravating factor of those effects.10
The type-1 herpes simplex virus (HSV-1) has an important role on the severity of chemotherapy-induced buccal mucositis in patients with hematological neoplasms,10 - 12 as do the Epstein-Barr virus (EBV) and the cytomegalovirus (CMV), representing a threat to immunocompromised patients.13, 14
In the �80s, the first multicenter protocol for the treatment of childhood ALL (GBTLI-80) was created in Brazil and it is currently in its sixth version (GBTLI-2009). This protocol has been used in about 14 treatment centers in Brazil, one of which is the Amazon Hematology and Hemotherapy Foundation (HEMOAM).
The HEMOAM is the only public reference service for treatment of childhood leukemia in the Northern region, with an annual demand of approximately 40 new ALL cases.15 Due to the lack of publications related to the profile of ALL patients in treatment with GBTLI ALL-2009,5 this study aims to evaluate the buccal mucosa of such patients regarding its clinical aspect and expression of the Herpesviridae (HHV) family members in several moments of the adopted chemotherapy protocol.
Material and methods
This research represents a case series study composed of nine consecutive cases of children and adolescents diagnosed with ALL, of both genders, with ages ranging from 2 to 14 years, from September 2014 to January 2015 in the HEMOAM Foundation. The procedures of this study follow the guidelines established by the ethics committee of the Federal University of Amazonas (UFAM protocol: 30934514.9.0000.5020) and HEMOAM (Protocol: 30934514.9.3001.0009).
A clinical analysis was performed to search for possible alterations of the lips, cheek mucosa, tongue, floor of the mouth, hard and soft palate, oropharynx, gums and surrounding regions, using a mouth mirror, a wooden spatula, gauze and proper biosecurity equipment. When buccal mucositis was observed, it was graded according to the Oral Toxicity Scale of the World Health Organization (WHO),16 where grades 1 and 2 correspond to moderate mucositis and grades 3 and 4 to severe mucositis.17
For the evaluation of HSV-1, EBV and CMV, samples were collected from the buccal mucosa and submitted to PCR analysis regardless of the presence of buccal mucositis. The patient�s mouth rinsed with sterile water and then the samples were obtained via oral swabs from the cheek mucosa (from molars to incisors), bilaterally, after brushing the mucosa with sterile cervical brushes (KolplastR Commercial Industrial do Brasil Ltda) from 5 to 10 seconds.10 The buccal evaluation and swabs were planned to be performed on days 0/1 (D0/D1), 8 (D8), 15 (D15) and 35 (D35) of the pre-phase and induction phase (P/I) and days 1(D1), 15 (D15), 29 (D29) and 50 (D50) of the consolidation of remission phase (CR).
The samples were transferred to 1.5 mL microtubes with 500 μL of TE buffer (10 mM Tris HCl and 1 mM EDTA, pH 8.0) and stored at -20oC. For the DNA extraction, 500 μL of TPK (10 mg/mL proteinase K, plus TE buffer and Tween 20%) were added to the tubes. The microtubes were briefly submitted to a vortex to homogenize the mix and then were incubated at 56�C for an hour, followed by ten more minutes at 100�C to activate the proteinase K. Afterwards, the DNA concentration was obtained via absorbance reading at 260 nm. The microtubes were stored at -20�C.
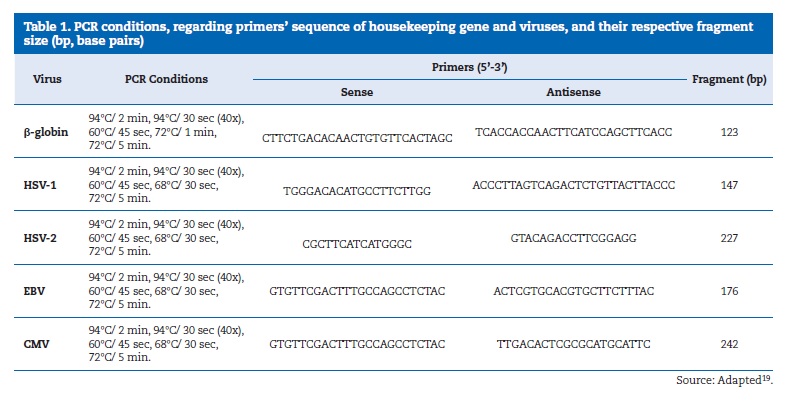
Specific sets of initiation pairs were used for the amplification and detection of HSV-1, EBV and CMV via PCR analysis.19 The same samples were submitted to PCR for the β-globin constitutive gene to control the occurrence of false-positive results. The positive controls for HSV-1, EBV and CMV were provided by the Tropical Medicine Foundation of Amazonas.
Ultrapure water associated with the reaction�s reagents was used for negative controls.
The PCR reactions for β-globin, HSV-1, EBV and CMV were performed in PCR conditions and primers according to (Table 1).
�
�
All of the PCR reactions were performed in Thermal Cycler (Applied BiosystemsR VeritiR Thermal Cycler). The amplicons were analyzed by electrophoresis in 2% agarose gel, stained with 0.8 μL of ethidium bromide (1μg/μL), in TBE 1X (tris + boric acid 0.089 M and EDTA 0.02 M) and visualized in a transilluminator under UV light, under electrical tension of 80 to 100 Volts. The size of amplified PCR fragments was determined by comparison with molecular weight markers (100 bp ready-to-use DNA Ladder, BioronR).
Results
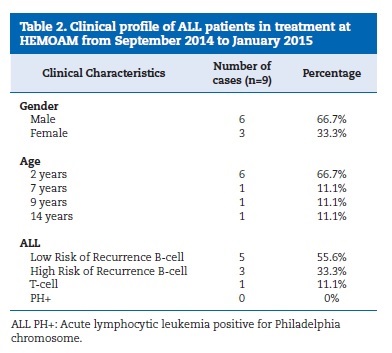
From September 2014 to January 2015, nine consecutive ALL cases of children and adolescents were analyzed, with a prevalence peak at around 2 years of age (66.7%), in males (66.7%) and with B-cell ALL diagnosis (55.6%). Clinical data regarding gender, age and subtype of ALL from the nine cases can be found in Table 2.
�
�
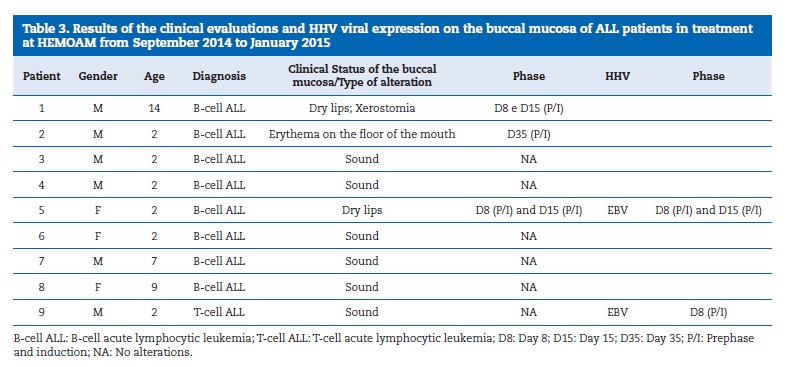
None of the patients with ALL presented buccal mucositis, but two patients presented dry lips on the 8th and 15th days of the induction phase and one of them also complained of xerostomia.
A third patient presented erythema on the floor of the mouth on the 35th day of the same chemotherapy phase.
None of the 23 samples gathered for the screening of HSV-1 and CMV were positive. On the other hand, three samples (33.3%) showed positive DNA amplification for EBV. The results of this study are assembled in Table 3.
�
�
Discussion
ALL is the most common malignant neoplasm in childhood and adolescence.1 - 3 It derives from hematopoietic cells and is characterized by increased malignant proliferation of lymphoid progenitor cells.20 Leukemia can be subclassified as acute or chronic. In its acute form, there is a fast increase in the number of immature cells circulating, impairing the bone marrow regards the production of healthy blood cells. In its chronic form, there is an excessive number of mature but abnormal white blood cells in the blood.21 It is frequently chemosensitive,4 but the chemotherapy may cause stomatological side effects that can be eased by oral monitoring of the patients throughout the entire treatment.2
The nine patients evaluated had 2 to 14 years of age, with a prevalence peak at 2-year-old male kids (66.7%), which confirms the literature statements that ALL affects children and adults, with a prevalence peak at 2 to 5 years of age.1 In one of the published researches, 24 men and 19 women were evaluated and, of those, 23 (53.5%) patients ranged from 3 to 6 years of age, with the most prevalent patients being females with 6 to 9 years of age.9 Another study with 42 patients pointed to 54.8% (n=23) female patients, with a mean age of 7.1 (� 4.7) years (median 5, minimum 2 and maximum 18 years), and distributed the patients regarding the age at diagnosis in groups of 2 (n=7; 16.7%), 4 (n=8; 19.0%) and 5 (n=4; 9.5%) years.8 The most affected gender in the present study counteracts the literature. Regarding age, some authors are unanimous when affirming that the age at diagnosis has a strong effect over the prognosis, with more favorable outcomes for younger patients.4, 22
Previous studies investigating the buccal mucosa of patients in treatment for ALL highlighted secondary buccal complications commonly occurring in the induction phase of the chemotherapy treatment, with a gradual decline over the following weeks.23 The most common complications are mucositis, candidiasis, periodontitis, gingivitis, ulcers, petechiae, ecchymosis, erythema, gingival bleeding, xerostomia, mucosa pallor and leukemic gingival enlargement.7 - 9, 23 However, in a prospective evaluation from diagnosis to the 10th week of treatment, alterations in saliva and lip mucosa were observed, because of the occurrence of severe buccal mucositis throughout almost all of the evaluation weeks.8 In the present study, only a few patients presented buccal alterations, contrasting with previous reports. The clinical findings of erythema (D35 P/I), dry lips (D8 and D15 P/I) and xerostomia (D15 P/I) are reported by most of the literature.6 - 9, 23 Although buccal mucositis is a common side effect of chemotherapy,10, 18, 23 it was not observed in the present case series, probably because of the small number of cases studied.
The relationship between HHV and hematological malignancies has been extensively studied, considering that it can be reactivated under immunosuppression.10, 24, 25 Studies have reported the seroprevalence of HSV-1, EBV and CMV, 25, 26 as well as the detection of DNA from those viruses in the buccal mucosa of ALL patients, which suggest that HSV-1 may be related to the severity of chemotherapy-induced buccal mucositis,10, 12 and that EBV may be associated to many kinds of epithelial and lymphoid malignancies.14 However, a research that evaluated the role of CMV and EBV in childhood B- and T-cell ALL pathogenesis reported that less than 20% of the samples were positive for at least one of the tested viruses and that the positive samples showed low infection levels;14 nevertheless, both represent a threat to immunocompromised patients, leading to morbidity and mortality among this group.13, 14 In comparison, in this study, after evaluation by qualitative PCR to search for HSV-1, CMV and EBV, no samples were positive for HSV-1 and CMV and three presented DNA amplification for EBV.
These results highlight the literature statements that EBV occurs later in life in developed countries,27 but mainly during childhood in developing countries.28
For more precise results, real-time PCR, as well as more advanced technologies, could aid in detecting those viruses more accurately, because some may remain in a latent state of the infection with low levels of viral genic expression, being barely detectable.19 Despite the limitation of access to molecular biology techniques, more studies need to be performed for a deeper analysis and more cases need to be prospectively followed-up, with constant monitoring, to gather more expressive data.
Conclusions
Taking into account the limitations of this case series, it can be concluded that the buccal alterations and the presence of HSV-1, CMV and EBV in the evaluated ALL patients were inexpressive, with most of the patients presenting sound mucosa throughout the treatment, without occurrences of mucositis.
It cannot be confirmed that the analyzed viruses integrate the microbiome of those patients. However, there are indications that the presence of EBV is more likely than HSV-1 and CMV, especially among child populations in developing countries, such as Brazil.
�
Refer�ncias
1. Stanulla M, Schrappe M. Treatment of Childhood Acute Lymphoblastic Leukemia. Semin Hematol. 2009;46:52-63.
2. Silverman LB, Stevenson KE, O�Brien JE, Asselin BL, Barr RD, Clavell L, et al. Long-term results of Dana-Farber Cancer Institute ALL Consortium protocols for children with newly diagnosed acute lymphoblastic leukemia (1985-2000). Leukemia. 2010;24:320-34.
3. Steliarova-Foucher E, Colombet M, Ries LAG, Moreno F, Dolya A, Bray F, et al. International incidence of childhood cancer, 2001�10: a population-based registry study. Lancet Oncol. 2017;18: 719-31.
4. Pui CH, Mullighan CG, Evans WE, Relling MV. Pediatric acute lymphoblastic leukemia: where are we going and how do we get there? Blood. 2012;120:1165-74.
5. Sociedade Brasileira de Oncologia Pediatrica. Protocolo Brasileiro de tratamento da leucemia linfoide aguda na infancia GBTLI LLA-2009. Sao Paulo: Campinas; 2011;1-347.
6. Brown CG, Wingard J. Clinical consequences of oral mucositis. Semin Oncol Nurs. Semin Oncol Nurs. 2004;20:16-21.
7. Toquica CPV, Silva PAM, Acero H. Caracterizacion clinicoepidemiologica de los pacientes pediatricos con leucemias agudas en la Clinica Universitaria Colombia. Serie de casos 2011-2014. Pediatr. 2016;49:17-22.
8. Ribeiro ILA, Limeira RRT, Castro RD, Bonan PRF, Valenca AMG. Oral Mucositis in Pediatric Patients in Treatment for Acute Lymphoblastic Leukemia. Int J Environ Res Public Health. 2017;28;14:1468.
9. Aggarwal A, Pai KM. Orofacial Manifestations of Leukemic Children on Treatment: A Descriptive Study. Int J Clin Pediatr Dent 2018;11:193-8.
10. Mendonca RM, de Araujo M, Levy CE, Morari J, Silva RA, Yunes JA et al. Prospective evaluation of HSV, Candida spp., and bucal bacteria on the severity of oral mucositis in pediatric acute lymphoblastic leukemia. Support Care Cancer. 2012;20:1101-7.
11. Chen YK, Hou HA, Chow JM, Chen YC, Hsueh PR, Tien HF. The impact of oral herpes simplex virus infection and candidiasis on chemotherapy-induced oral mucositis among patients with hematological malignancies. Eur J Clin Microbiol Infect Dis. 2011;30:753-9.
12. Ruping MJ, Keulertz C, Vehreschild JJ, Lovenich H, Sohngen D, Wieland U et al. Association of HSV reactivation and pro-inflammatory cytokine levels with the severity of stomatitis after BEAM chemotherapy and autologous SCT. Support Care Cancer. 2011;19:1211-6.
13. Halenius A, Hengel H. Human cytomegalovirus and autoimmune disease. Biomed Res Int. 2014;1-15.
14. Chen CY, Huang KY, Shen JH, Tsao KC, Huang YC. A largescale seroprevalence of epstein-barr virus in Taiwan. Plos One. 2015;10:1-11.15. Salina TD, Ferreira YA, Alves EB, Ferreira CM, De Paula EV, Mira MT et al. Role of peripheral blood minimum residual disease at day 8 of induction therapy in high-riskpediatric patients with acute lymphocytic leukemia. Sci Rep. 2016; 6:31179.
16. World Health Organization, 1979. Handbook for Reporting Results of Cancer Treatment. World Health Organization, Switzerland: Geneva, 1979. E-book available on https://apps.who.int/iris/handle/10665/37200 Access in June/2019.
17. Otmani N, Alami R, Hessissen L, Mokhtari A, Soulaymani A, Khattab M. Determinants of severe oral mucositis in paediatric cancer patients: a prospective study. Int J Paediatr Dent. 2011;21:210-6.
18. Pinto ETF, Queiroz SIML, Gon�alves PGP, Gurgel BCV. Avaliacao retrospectiva das altera��es orais em crian�as com leucemia linfoblastica aguda. Rev Port Estomatol Med Dent Cir Maxilofac. 2018;59:30-5.
19. Morales-Sanchez A, Pompa-Mera EN, Fajardo-Gutierrez A, Alvarez-Rodriguez FJ, Bekker-Mendez VC, Flores-Chapa JD et al. EBV, HCMV, HHV6 and HHV7 screening in bone marrow samples from children with acute lymphoblastic leukemia. Biomed Res Int. 2014;2014:1-10.
20. Ladines-Castro W, Barragan-Ibanez G, Luna-Perezb MA, Santoyo-Sanchez A, Collazo-Jaloma J, Mendoza-Garcia E et al. Morphology of leukaemias. Rev Med Hosp Gen Mex. 2016;79:107-13.
21. An Q, Fan CH, Xu SM. Recent perspectives of pediatric leucemia � an update. Eur Rev Med Pharmacol Sci. 2017;21:31-6.
22. Pui CH, Pei D, Campana D, Bowman WP, Sandlund JT, Kaste SC et al. Improved prognosis for older adolescents with acute lymphoblastic leukemia. J Clin Oncol.
2011;29:386-91.
23. Morais EF, Lira JA, Macedo RA, Santos KS, Elias CT, Morais M de L. Oral manifestations resulting from chemotherapy in children with acute lymphoblastic leukemia. Braz J Otorhinolaryngol. 2014;80:78-85.
24. Nefzi F, Lambert C, Gautheret-Dejean A, Fisson S, Khebizi Q, Khelif A et al. Cytokine and cellular responses to human herpesvirus-6B in patients with B-acute lymphoblastic leukemia. Microbiol Immunol. 2016;60:770-7.
25. Loutfy SA, Alam El-Din HM, Ibrahim MF, Hafez MM. Seroprevalence of herpes simplex virus types 1 and 2, Epstein-Barr virus, and cytomegalovirus in children with acute lymphoblastic leukemia in Egypt. Saudi Med J. 2006;27:1139-45.
26. Santos de Faria AB, Silva IH, de Godoy Almeida R, Silva SP, Carvalho AT, Leao JC. Seroprevalence of herpes virus associated with the presence and severity of oral mucositis in children diagnosed with acute lymphoid leukemia. J Oral Pathol Med. 2014;43:298-303.
27. Grotto I, Mimouni D, Huerta M, Mimouni M, Cohen D, Robin G et al. Clinical and laboratory presentation of EBV positive infectious mononucleosis in young adults. Epidemiol Infect. 2003;131:683-9.
28. Oliveira JL, Freitas RT, Arcuri LJ, Gomes AP, Vitorino RR, Rodrigues DC et al. O virus Epstein-Barr e a mononucleose infecciosa. Rev Bras Clin Med. Sao Paulo, 2012;10:535-43.
�
Tatiana Nayara Liborio Kimura
E-mail address: tatiana.liborio@gmail.com
�
Acknowledgements
This study received financial support from the National Council for Scientific and Technological Development (CNPq/Grant:486348/2013-0). The authors thank the Coordination for the Improvement of Higher Education Personnel (CAPES), the Ministry of Education of Brazil for the financial support to the Postgraduate Program in Dentistry at the Federal University of Amazonas, the Molecular Diagnostic Laboratory and Molecular Support Center of UFAM, the technical support from HEMOAM (Celia Maria Bolognese Ferreira and Cristina Motta Ferreira), and the Foundation of Tropical Medicine (Dr. Luiz Carlos de Lima Ferreira). We also thank Renata Gualberto da Cunha for the technical collaboration.
�
Ethical disclosures
Protection of human and animal subjects. The authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of data. The authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consent. The authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
�
Conflict of interest
The authors have no conflicts of interest to declare.
�
Article history:
Received 18 September 2019
Accepted 6 December 2019
Available online 3 January 2020
