
Revista Portuguesa de Estomatologia, Medicina Dentária e Cirurgia Maxilofacial
SPEMD | 2019 | 60 (4) | 155-162
Original research
Surface properties after chemical aging of chlorhexidine delivery systems based on acrylic resin
Propriedades de superfície após envelhecimento químico de sistemas de libertação de clorexidina à base de resina acrílica
a Faculdade de Medicina Dentária, Universidade de Lisboa, Lisbon, Portugal
b Research Institute for Medicines (iMed.ULisboa), Faculdade de Farmácia, Universidade de Lisboa, Lisbon, Portugal
c Oral and Biomedical Sciences Research Unit (UICOB), Faculdade de Medicina Dentária, Universidade de Lisboa, Lisbon, Portugal
Cristina Bettencourt Neves - cristina.neves@fmd.ulisboa.pt
Article Info
Rev Port Estomatol Med Dent Cir Maxilofac
Volume - 60
Issue - 4
Original research
Pages - 155-162
Go to Volume
Article History
Received on 13/08/2019
Accepted on 05/12/2019
Available Online on 20/12/2019
Keywords
Original research
�
Surface properties after chemical aging of chlorhexidine delivery systems based on acrylic resin
Propriedades de superf�cie ap�s envelhecimento qu�mico de sistemas de liberta��o de clorexidina � base de resina acr�lica
�
Joana Costa a, Ana Bettencourt b, Ana Madeira a, Lu�s Nepomuceno a, Jaime Portugal c, Cristina Bettencourt Neves c,*
a Faculdade de Medicina Dentaria, Universidade de Lisboa, Lisbon, Portugal
b Research Institute for Medicines (iMed.ULisboa), Faculdade de Farmacia, Universidade de Lisboa, Lisbon, Portugal
c Oral and Biomedical Sciences Research Unit (UICOB), Faculdade de Medicina Dentaria, Universidade de Lisboa, Lisbon, Portugal
�
�
http://doi.org/10.24873/j.rpemd.2019.12.688
�
Abstract
Objectives: To evaluate the effect of chlorhexidine incorporation on the surface free energy and microtensile bond strength of three reline acrylic resins, after chemical aging.
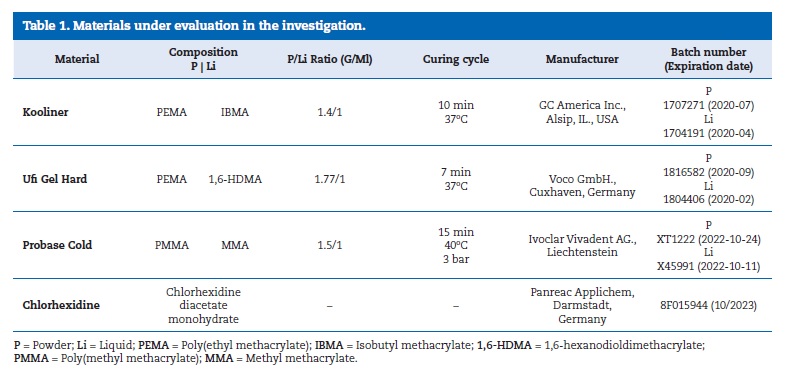
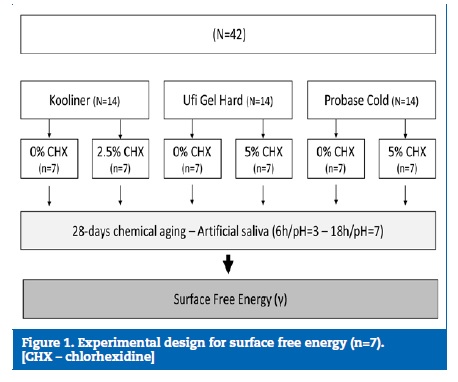
Methods: For each of the studies, six experimental groups were set according to reline resin and chlorhexidine incorporation [Kooliner � 0% vs. 2.5% chlorhexidine; Ufi Gel Hard and Probase Cold � 0% vs. 5% chlorhexidine]. The specimens were submitted to a chemical aging process for 4 weeks (pH fluctuation in artificial saliva, cycles of 6 hours at pH=3 and 18 hours at pH=7). For the first study, 42 specimens were prepared (n=7) and, after chemical aging, the surface free energy was calculated. For the adhesive strength study, 36 denture base resin cubes were prepared and reline resin was applied to them according to the experimental group (n=6). Five sticks (1�1 mm section) were obtained from each specimen and submitted to chemical aging followed by microtensile test (1 kN; 1 mm/min). Data were analyzed with Kruskal-Wallis and Mann-Whitney non-parametric tests (α=0.05).
Results: Differences (p<0.05) were observed between resins, both in surface energy and in bond strength. The chlorhexidine incorporation did not affect the surface energy of neither of the resins and also did not affect the bond strength of Kooliner and Ufi Gel Hard (p>0.05). Incorporating 5% chlorhexidine (p=0.004) decreased the Probase Cold bond strength to the denture base resin.
Conclusions: After chemical aging, chlorhexidine incorporation only negatively affected the bond strength of Probase Cold.
Keywords: Acrylic resins, Chlorhexidine, Denture stomatitis, Surface tension, Tensile strength
�
Resumo
Objetivos: Avaliar o efeito da incorpora��o de clorexidina na energia de superf�cie e resist�ncia adesiva � microtra��o de tr�s resinas acr�licas de rebasamento, ap�s envelhecimento qu�mico.
M�todos: Para cada um dos estudos foram criados 6 grupos experimentais de acordo com resina de rebasamento e incorpora��o de clorexidina (Kooliner � 0% vs. 2,5%; Ufi Gel Hard e Probase Cold � 0% vs. 5%). Os esp�cimes foram submetidos a um processo de envelhecimento qu�mico durante 4 semanas (varia��es de pH em saliva artificial, com ciclos de 6 horas em pH=3 e 18 horas em pH=7). Para o primeiro estudo foram preparados 42 esp�cimes (n=7) e ap�s envelhecimento qu�mico foi calculada a energia de superf�cie. Para determina��o da resist�ncia adesiva, foram preparados 36 cubos de Probase Hot e sobre estes aplicada a resina de rebasamento de acordo com o grupo experimental (n=6). Obtidos 5 palitos (sec��o 1�1 mm) de cada esp�cime, foram sujeitos a envelhecimento qu�mico, seguido de teste de microtra��o (1 kN; 1 mm/min). Os dados foram avaliados estatisticamente com testes n�o-param�tricos segundo Kruskal-Wallis e Mann-Whitney (α=0,05).
Resultados: Observaram-se diferen�as (p<0,05) entre as resinas, tanto na energia de superf�cie como na resist�ncia adesiva. A energia de superf�cie de nenhuma das resinas foi afetada pela incorpora��o de clorexidina, que tamb�m n�o afetou a resist�ncia adesiva de Kooliner e de Ufi Gel Hard (p>0,05). Incorporar 5% de clorexidina reduziu significativamente (p=0,004) a resist�ncia adesiva de Probase Cold.
Conclus�es: Ap�s envelhecimento qu�mico, a incorpora��o de clorexidina apenas afetou negativamente a resist�ncia adesiva de Probase Cold.
Palavras-chave: Resinas acr�licas, Clorexidina, Estomatite prot�tica, Tens�o superficial, Resist�ncia � tra��o
�
Introduction
The physiologic progression of a residual ridge resorption can affect the adaptation and retention of a denture base and lead to loss of masticatory efficiency and comfort, as well as possible trauma of the underlying tissues.1, 2 In order to avoid denture rejection, periodic examination of the denture and underlying support tissues is advised to detect these changes and, if necessary, a relining procedure should be performed.3, 4
Denture stomatitis is a highly prevalent chronic condition, usually asymptomatic, that manifests as a diffuse inflammation of the mucosa in contact with the denture.5 - 8 Despite the evidence of fungal etiology, several factors have been suggested in a multifactorial etiology.9 Even though other Candida species may contribute to this disease, Candida albicans is the main causative agent, and its adherence to the oral mucosa and the denture surface is considered the first step in the pathogenesis of denture stomatitis.9
Treatment usually involves topical or systemic antifungal therapy, good oral hygiene, denture cleaning procedures, adjustment of denture failures, discontinuation of night-time denture wear, nutritional restitution and relining or replacing of the denture.7, 10 - 17 Topical and systemic antifungal therapy is commonly applied, in spite of contributing to highly frequent relapse episodes of the disease, since the maintenance of the therapeutic dosage of the drugs is very dependent on complex regimes of patient compliance. Also, antifungal therapy does not provide the complete eradication of the microorganisms from the dentures surface. 16 - 19 Chlorhexidine (CHX), an antimicrobial agent that acts against a wide range of microorganisms, including Candida species, is another possible therapy.14 - 17 However, its efficiency in topical solutions depends on the turnover of saliva and the cleansing action of the oral musculature.14, 20 In order to increase the availability of the agent in the target area at a therapeutic dosage, loading reline acrylic resins with CHX has been proposed as a therapeutic approach for denture stomatitis, to allow a slow and sustained releasing for at least 28 days, with a more effective antifungal activity than other drugs.14 - 18, 21, 22
Reline acrylic resins may have different chemical compositions and structural arrangements. Kooliner and Ufi Gel Hard, a non-crosslinking and a crosslinking relining material, respectively, are both poly(ethyl methacrylate)-based resins and are used in a direct technique, being polymerized in the mouth. On the other hand, Probase Cold, which is a poly(methyl methacrylate)-based reline material, is used in an indirect technique, being polymerized under laboratory conditions.23, 24
The incorporation of CHX into polymeric materials may affect their mechanical and surface properties.25 A CHX concentration of 2.5% for Kooliner and 5% for both Ufi Gel Hard and Probase Cold was established as the minimal concentration effective against Candida albicans, without affecting mechanical properties, both immediately after acrylic preparation and after thermal aging.17, 26 - 31 However, the effect of the chemical aging process on the resin properties may also be relevant since, when in function inside the oral cavity, reline acrylic resins may biodegrade faster due to daily exposure to an acidic environment.32, 34
The surface free energy of a solid consists in the sum of components arising from dispersive (apolar) and polar contributions. Changes in this property will impact the surface wettability of the material and, consequently, its interaction with saliva molecules, microbial molecules and other materials.13, 35 Also, an adequate bonding between the denture base resin and the reline material is essential since a failure can harbor bacteria, promote staining, decrease the strength of the denture and cause fractures.11, 16 - 39
The objective of this study was to evaluate the impact of incorporating CHX in reline acrylic resins subjected to oral pH fluctuations on their surface free energy and their microtensile bond strength to the denture base resin. The following hypotheses were established: 1) no differences in surface free energy and bond strength are observed between the three resins; 2) CHX incorporation does not influence the surface free energy; and 3) incorporating CHX in the reline resins does not affect their bond strength to the denture base.
Materials and methods
Three auto-polymerizing reline acrylic resins were selected: two direct reline resins mainly formed by poly(ethyl methacrylate) � Kooliner and Ufi Gel Hard, and an indirect reline resin mainly composed by poly(methyl methacrylate) � Probase Cold.
The acrylic resins were manipulated according to the manufacturer�s recommendations ((Table 1). The liquid was measured using a graduated pipette. The powder of each reline acrylic resin and the chlorhexidine diacetate monohydrate (Panreac Applichem, Darmstadt, Germany) were weighted using a precision balance (A&D FZ-200i Company, Limited, Tokyo, Japan), to obtain a 2.5%powder weight (w/w) for Kooliner and a 5% powder weight (w/w) for Ufi Gel Hard and Probase Cold.
�
�
They were then mixed with a mortar and a pestle until homogenization was achieved. Two groups were set for each material: one control group without CHX, and one experimental group with CHX incorporation.
For the surface free energy (γ) study, a metallic rectangular mold was used to prepare 42 specimens (n=7) with standardized dimensions (25x16x1 mm). After allowing the resin to polymerize under pressure, the specimens were polished with a 600-grit silicon carbide paper (Carbimet Paper Discs, Buehler Ltd., Lake Bluff, IL). They were then submitted to a chemical aging process in a graduated falcon tube filled with artificial saliva, with a ratio of 1 g / 5 mL, in an incubator (Memmert, Schwabach, Germany) at 37 � 2oC with constant gentle shaking (300 rpm).40, 41 The specimens were exposed to pH fluctuations in cycles of 6 hours in saliva at pH=3 and 18 hours at pH=7, for 28 days. Between each cycle, specimens were washed with distilled water and dried with absorbent paper ((Figure 1).
�
The γ test was performed with a Tensiometer K12 (Kruss, Hamburg, Germany), where the specimen was suspended and immersed 4 mm in water (Merck Millipore, Germany) and 1,2-propanediol (1-2 Propanediol R.822324-1L; Merck, Germany) at a speed of 20 μms-1. The contact angles were measured at 25 � 0.1oC using the Wilhelmy plate technique, and were used to estimate total surface free energy (γ), as well as its dispersive (γd) and polar components (γp), based on the harmonic mean method proposed by Wu.42, 43
For the microtensile bond strength (μTBS) study, 36 cubic (10�10�10 mm) specimens of the heat-polymerizing denture base acrylic resin Probase Hot (Ivoclar Vivadent AG, Liechtenstein) were produced by a conventional flasking technique.
These specimens were submitted to 2500 thermal cycles, alternating submersions of 20 seconds at 5oC and 55oC with a duel time of 5 seconds, on a thermocycling machine (Refri 200-E, Aralab, Cascais, Portugal), in order to simulate a 3-month aging process inside the oral cavity.44> The surfaces of the denture base specimens were finished to a 3-mm thickness in a rotational grinding and polishing machine (DAP-U, Struers, Denmark) with a 600-grit silicon carbide paper (Carbimet Paper Discs, Buehler Ltd., Lake Bluff, IL). Their thickness was confirmed using a digital micrometer (Mitutoyo Digimatic, MFG. Co., Ltd. Tokyo, Japan) with � 0.01-mm precision.
Afterward, the relining procedure was performed. The surface of the Probase Hot was previously conditioned according to the reline resin used. For Kooliner and Probase Cold, the bonding area was scrubbed once with a microbrush soaked with the correspondent monomer. In Ufi Gel Hard groups, a specific conditioner was applied to the area and let dry for 30 seconds, as recommended by the manufacturer. Then, the freshly mixed reline resin was placed on the bonding area.
After polymerization, five sticks with a section of 1 mm2 were obtained from each specimen, using an Isomet cutting machine 1000 Precision Saw (Serial No. 666-IPS-03518; Buehler, Lake Bluff, IL, USA), under constant water refrigeration. The sticks were identified, submitted to an aging process in artificial saliva by previously described methods, and tested. The μTBS test was performed with a universal testing machine (Instron model 4502, Instron Ltd., Bucks, HP 12 3SY, England) using a 1 kN load cell and a 1 mm/min crosshead speed, until fracture ((Figure 2).
�
The failure mode was assessed by two calibrated observers with a stereomicroscope and classified as adhesive, cohesive or mixed: adhesive if it occurred between the reline resin and the denture base resin, cohesive when it occurred exclusively within one of the resins, and mixed if it occurred in the interface of the two resins but included residues of reline resin.
Since normality was not verified (Shapiro‑Wilk normality tests, p<0.05), γ and μTBS data were submitted to Kruskal-Wallis and Mann‑Whitney nonparametric tests (α=0.05). Failure mode data were analyzed with chi-square tests (α=0.05).
Results
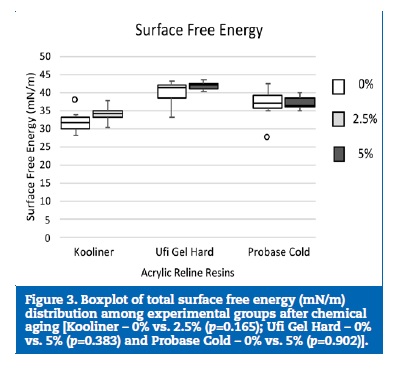
The total surface free energy values ((Table 2) ranged between 41.9 mN/m, in the Ufi Gel Hard loaded with 5% CHX, and 32.1 mN/m, in the Kooliner without CHX. The three reline acrylic resins presented statistically significantly (p<0.001) different total surface free energy values.
�
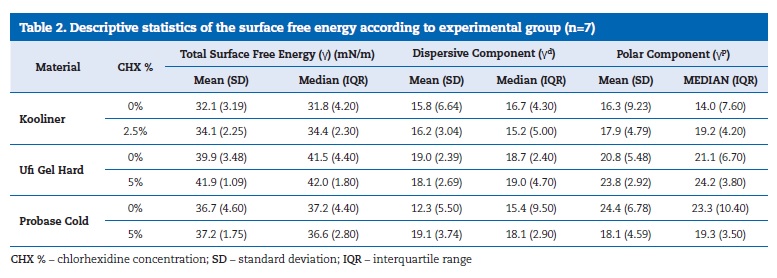
The surface energy of the Kooliner specimens was lower than both Probase Cold (p=0.046) and Ufi Gel Hard (p<0.001) specimens. Also, Probase Cold showed higher surface energy values than Ufi Gel Hard (p=0.035). Regarding the effect of the CHX incorporation, no statistically significant influence was detected in the three reline resins (Kooliner, p=0.165; Ufi Gel Hard, p=0.383; Probase Cold, p=0.902) ((Figure 3).
�
�
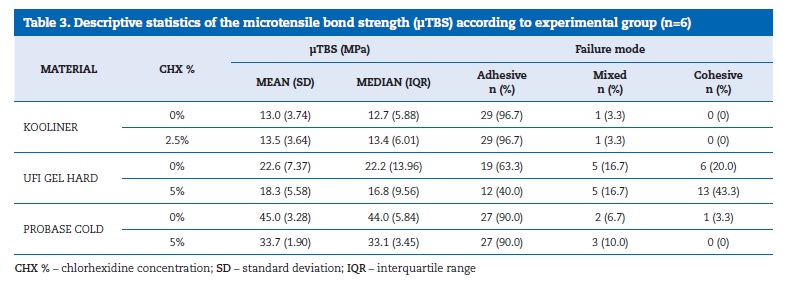
The μTBS values ((Table 3) ranged between 45.0 MPa, in the Probase Cold without CHX, and 13.0 MPa, in the Kooliner without CHX. Probase Cold specimens yielded a higher μTBS than Kooliner (p<0.001) and Ufi Gel Hard (p=0.004) specimens. No difference (p=0.144) was found between Kooliner and Ufi Gel Hard groups.
�
�
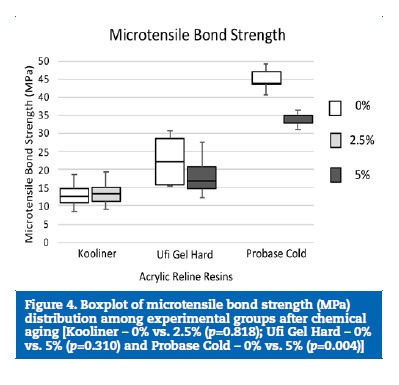
Neither Kooliner (p=0.818) nor Ufi Gel Hard (p=0.310) bond strength to the denture base was affected by CHX incorporation. However, loading Probase Cold with 5% of CHX resulted in lower (p=0.004) μTBS values than the control group (Figure 4).
�
�
The failure mode was predominantly adhesive (79.4%) and was not affected by CHX incorporation (Kooliner, p=1.000; Ufi Gel Hard, p=0.125; Probase Cold, p=0.549).
Discussion
Exposing reline acrylic resins to oral pH fluctuations may affect their physical and biomechanical properties.40, 45, 46
Also, the maximum cumulative release of CHX reaches higher levels at pH=3 than at pH=7.31, 47 However, although all specimens in the present study were immersed in artificial saliva with a cyclic procedure of 6 hours at pH=3 interchanging with 18 hours at pH=7 for 28 days, the incorporation of CHX in the reline acrylic resins studied only affected the microtensile bond strength of the Probase Cold to the denture base.
Since differences were found between the surface free energy and microtensile bond strength of the three reline acrylic resins, the first null hypothesis is rejected. These differences may result from the distinct chemical composition and structural arrangement of the three reline acrylic resins.23, 24 Kooliner had lower total surface free energy values than Ufi Gel Hard and Probase Cold, probably because of its surface�s porous structure. Air voids are entrapped when mixing the powder and liquid components as a consequence of a rapid polymerization reaction.48, 49 Regarding microtensile bond strength, Probase Cold presented higher values than the other tested resins, probably because its chemical composition is similar to the denture base resin: they are both based on polymethylmethacrylate (PMMA) polymer and have methyl methacrylate (MMA) as the monomer. This similarity allows a smooth diffusion of the reline monomers into the denture base resin and forms a complex and strong network in the interface. On the other hand, the lowest mean values of microtensile bond strength were obtained in both the control and the 2.5%-CHX loaded Kooliner. This result may be due to the composition of its monomer isobutyl methacrylate, a high-molecular-weight monomer that makes the dissolution of the PMMA in the denture base resin surface difficult and leads to a less effective penetration of the reline resin into the denture base.24 Similar results were found in previous studies when the three resins were submitted to thermal aging conditions.27 - 30
Loading the three reline acrylic resins with CHX did not change their total surface energy after chemical aging. So, the second hypothesis is not rejected. Similar results were found in a previous study where the same percentage of CHX incorporation showed a null effect on total surface energy after thermal aging.34 However, it seems that thermal aging in water leads to lower total surface free energy than chemical aging in saliva (the method used in the present study), probably because of the different aging environments.34, 35
No differences were found in the microtensile bond strength values between control and experimental groups of Kooliner and Ufi Gel Hard. Probase Cold was the only resin whose microtensile bond strength decreased, since the 5%-CHX group had lower values than the control. This indirect acrylic resin is composed of pre-polymerized poly(methyl methacrylate) particles, and it is polymerized with an indirect method, under high temperature and pressure. Those results may be explained by the incorporation of CHX within the polymer matrix of the material, which probably led to higher distances between the molecules of the polymer net and less homogeneity in their structural arrangement, therefore weakening the bond strength.16 Nevertheless, Probase Cold specimens loaded with 5% CHX presented higher values of μTBS than any of the other materials studied. Thus, the third null hypothesis is rejected.
Regarding the failure modes, the most observed failure in Kooliner was adhesive in both groups, justified by the distinct chemical composition of this reline resin and the denture base polymer, and proved by the lower μTBS values observed in the present study. Concerning Ufi Gel Hard, the most frequent failure mode was cohesive, probably caused by an increase of the bond strength promoted by the specific adhesive used before the relining procedure. Also, the cohesive failures on this resin were higher in the experimental group than in the control group. Thus, the presence of CHX may weaken the internal structure of the Ufi Gel Hard and promote failures in the polymer instead of in the bond interface. Both control and experimental groups of Probase Cold showed a predominance of adhesive failures. The robust and complex network formed by PMMA molecules and their strong internal structure can explain the absence of cohesive failures on Probase Cold.50, 51
Although microhardness and flexural strength after chemical aging of chlorhexidine delivery systems have already been studied,52 other properties should be evaluated, and more laboratory studies that mimic the oral environment and clinical trials are needed.
Conclusions
The incorporation of 5% chlorhexidine in Probase Cold resin negatively affected its microtensile bond strength to the denture base, after a chemical aging correspondent to 1 month of exposure to the oral environment. No other negative effects were observed on the surface free energy and bond strength of the three reline acrylic resins loaded with the studied percentage of chlorhexidine.
�
Refer�ncias
1. Van Meegen H, Kalk W. Improvement Of A Removable Complete Denture By Relining Or Rebasing. Ned Tijdschr Tandheelkd. 2011;118:545-51.
2. Tallgren A. The continuing reduction of the residual alveolar ridges in complete denture wearers: a mixed- longitudinal study covering 25 years. Roman;mso-bidi-font-family: Arial;color:#0D0D0D;mso-themecolor:text1;mso-themetint:242'>J Prosthet Dent. 1972;27:121-32.
3. Leles C, Machado A, Vergani C, Giampaolo E, Pavarina A. Bonding Strength Between A Hard Chairside Reline Resin And A Denture Base Material As Influenced By Surface Treatment. J Oral Rehabil. 2001;28:1153-7.
4. Reis J, Vergani C, Pavarina A, Giampaolo E, Machado A. Effect of relining, water storage and cyclic loading on the flexural strength of a denture base acrylic resin. J Dent. 2006; 34:420-6.
5. Reichart P. Oral mucosal lesions in a representative cross-sectional study of aging Germans. Community Dent Oral Epidemiol. 2000;28:390-8.
6.
Moskona D, Kaplan I. Oral
lesions in elderly denture wearers. 7.
Yarborough A, Cooper L, Dugum I, Mendonca
G, McGraw K, Stoner L. Evidence Regarding the
Treatment of Denture Stomatitis. Roman;mso-bidi-font-family:
Arial;color:#0D0D0D;mso-themecolor:text1;mso-themetint:242'>J Prosthodont. 2016 Jun;25:288-301. 8.
Da Silva P, Acosta E, Pinto L, Graeff M, Spolidorio D, Almeida R, Porto V. Microscopical
Analysis Of Candida albicans Biofilms On Heat-Polymerised Acrylic
Resin After Chlorhexidine Gluconate
And Sodium Hypochlorite Treatments.
Mycoses. 2011;54:712-7. 9.
Rautemaa R, Ramage G. Oral candidosis �
Clinical challenges of a biofilm disease. Crit
Rev Microbiol. 2011;
37:328-36.10.
Redding S, Bhatt B, Rawls H, Siegel G, Scott K, Lopez-Ribot
J. Inhibition of Candida albicans
biofilm formation on denture material. Oral
Surg Oral Med Oral Pathol
Oral Radiol Endod. 2009; 107:669-72. 11.
Alcantara C, Macedo A, Gurgel B, Jorge J, Neppelenbroek
K, Urban V. Peel Bond Strength Of Resilient Liner Modified By
The Addition Of Antimicrobial Agents To Denture Base
Acrylic Resin.
J Appl Oral Sci. 2012;20:607-12. 12.
Sousa F, Paradella T, Koga-Ito C, Jorge A. Effect of sodium bicarbonate on Candida albicans adherence to
thermally activated acrylic resin. Braz
Oral Res. 2009; 23:381-5. 13.
AL-Dwairi Z, AL-Quran F, AL-Omari
O. The effect of antifungal agents on surface
properties of poly(methylmethacrylate)
and its relation to adherence of Candida albicans.
J Dent Res. 2012; 56:272-80. 14.
Ryalat S, Darwish R, Amin
W. New Form Of Administering Chlorhexidine For Treatment Of Denture-Induced Stomatitis.
Ther Clin
Risk Manag. 2011;7:219-25. 15.
Amin W, Al-Ali M, Salim N, Al-Tarawneh
S. A New Form Of Intraoral
Delivery Of Antifungal Drugs For The Treatment Of Denture-Induced Oral Candidosis. Eur J Dent. 2009;3:257-66. 16.
Salim N, Silikas N, Satterthwaite J, Moore C, Ramage
G, Rautemaa R. Chlorhexidine-Impregnated
PEM/THFM Polymer Exhibits Superior Activity To
Fluconazole-Impregnated Polymer Against Candida albicans Biofilm Formation. Int J Antimicrob Agents. 2013;41:193-6. 17. Marcelino N, Barreiros M, Bettencourt A, Neves C. Efeito da Incorporacao de Clorexidina em Resinas
de Rebasamento � Estudos de Libertacao. Rev Port Estomatol
Med Dent Cir Maxilofac. 2015;56(S1):14. 18.
Salim N, Moore C, Silikas
N, Satterthwaite J, Rautemaa
R. Fungicidal Amounts Of Antifungals Are Released From
Impregnated Denture Lining Material For Up To 28 Days.
J Dent.2012;40:506-12. 19. Lima J, Maciel J, Hotta
J, Vizoto A, Honorio H, Urban V et al.
Porosity of temporary denture soft liners containing
antifungal agents.
J Appl
Oral Sci. 2016; 24:453-61. 20. Urban V, Machado A,
Oliveira R, Vergani C, Pavarina
A, Cass Q. Residual monomer of reline acrylic
resins. Effect
of water-bath and microwave post-polymerization treatments. Dent
Mater. 2007; 23:363-68. 21.
Bertolini M, Portela M, Curvelo A, Soares R, Lourenço
E, Telles D. Resins-Based
Denture Soft Lining Materials Modified By Chlorhexidine
Salt Incorporation: An In Vitro Analysis Of Antifungal
Activity, Drug Release And Hardness. Dent Mater. 2014;30:793-8. 22.
Cao Z, Sun X, Yeh C, Sun Y. Rechargeable
Infection-responsive Antifungal Denture Materials. J Dent Res. 2010; 89:1517-21. 23.
Arima T, Murata H, Hamada T. Properties
Of Highly Cross-Linked Autopolymerizing Reline
Acrylic Resins.
J Prosthet Dent. 1995;73:55-9. 24.
Arima T, Nikawa H, Hamada
T, Harsini. Composition and effect of
denture base resin surface primers for reline acrylic resins.
J Prosthet Dent.
1996; 75:457-62. 25.
Patel M, Cruchley A, Coleman D, Swai
H, Braden M, Williams D. A Polymeric System For The Intra-Oral Delivery Of An Anti-Fungal Agent.
Biomaterials. 2001;22:2319-24. 26. Costa J, Alexandre F, Bettencourt A, Ribeiro I,
Portugal J, Neves C.
Incorporação
De Clorexidina Em Resinas De Rebasamento
� Estudos Microbiológicos. Rev Port
Estomat Med Dent Cir Maxilofacial.
2017;58(S1):e51. 27. Sousa C, Costa J, Matos A, Bettencourt A, Portugal
J, Neves C. Efeito
Da Incorporacao De Clorexidina
Nas Propriedades De Resinas Acrilicas De Rebasamento. Rev Port
Estomatol Med Dent Cir Maxilofac.
2014;55(S1):e23-4. 28. Barreiros M, Marcelino N, Bettencourt A, Neves C. Incorpora��o de clorexidina em resinas de rebasamento
� Propriedades de superficie. Rev Port Estomatol
Med Dent Cir Maxilofac. 2015;56(S1):e14. 29. Costa N, Costa J, Portugal J, Bettencourt A, Neves
C.
Resinas acrilicas com clorexidina
envelhecidas � estudos de ades�o e energia de superficie. Rev Port
Estomatol Med Dent Cir Maxilofac. 2018;59(S1):e44. 30.
Rijo I, Pedro D, Costa J, Bettencourt A, Portugal J, Neves C. Chlorhexidine Loading Of Acrylic Reline
Resins � Microhardness And
Flexural Strength After Thermal Aging. Rev Port Estomatol Med Dent Cir
Maxilofac. 2018;59:154-61. 31. Alexandre F, Costa J, Goncalves
L, Bettencourt A, Neves C. Envelhecimento quimico e libertacao
de clorexidina em resinas acrilicas de rebasamento. Rev
Port Estomatol Med Dent Cir Maxilofac.
2017;58:51-52. 32.
Jepson N, McGill J, McCabe J. Influence Of Dietary
Simulating Solvents On The Viscoelasticity Of Temporary Soft Lining Materials. J Prosthet
Dent.
2000;83:25-31. 33.
Da Mata A, Marques D, Silveira J, Marques J, Felino E, Guilherme N.
Effects Of Gustatory Stimulants Of Salivary Secretion On Salivary pH And Flow: A Randomized
Controlled Trial. Oral Dis. 2009;15:220-8. 34.
Bettencourt A, Neves C, De Almeida M, Pinheiro L, Oliveira S, Lopes L, Castro M.
Biodegradation Of Acrylic Based Resins: A Review. Dent Mater. 2010;26:e171-80. 35.
Jin NY, Lee HR, Lee H, Pae A. Wettability of denture relining materials under
water storage over time. J Adv
Prosthodont. 2009; 1:1-5. 36. Pinto JR, Mesquita MF, Nobilo
MA, Henriques, GE. Evaluation of varying
amounts of thermal cycling on bond strength and permanent deformation of two
resilient denture liners. J
Prosthet Dent. 2004; 92:288-93. 37.
Mutluay MM, Ruyter IE. Evaluation of adhesion of chairside
hardre lining materials to denture base polymers. J Prosthet
Dent. 2005; 94:445-52. 38.
Takahashi Y, Chai J. Shear Bond Strength of
Denture Reline Polymers to Denture Base Polymers. Int
J Prosthodont. 2001;
14:271-75. 39.
Neppelenbroek K, Pavarina
A, Gomes M, Machado A, Vergani C. Bond strength of hard chairside
reline resins to a rapid polymerizing denture
base resin before and after thermal cycling. J Appl Oral Sci. 2006; 14:436-42. 40.
Preetha A, Banerjee R. Comparison
of Artificial Saliva Substitutes. Trends Biomater.
Artif.
Organs. 2005;18: 178-86. 41.
Bettencourt A, Feliz M, Sousa C, Goncalves
L, Neves C. An acrylic reline resin
loaded with chlorhexidine: Insights on drug release.
Rev Port Estomatol Med Dent Cir Maxilofac. 2016;57:
125-31. 42. Bettencourt A, Calado A, Amaral J, Alfaia A, Vale
F, Monteiro J et al. Surface studies on acrylic bone cement. Int
J Pharm. 2004; 278:181-6. 43.
Wu S. Calculation of interfacial tension in polymer
systems.
J Polym Sci
C Polym Symp. 2007; 34:19-30. 44.
Gale M, Darvell B. Thermal
cycling procedures for laboratory testing of dental restorations.
J Dent. 1999;27:89-99. 45.
Santerre JP, Shajii L,
Leung BW. Relation of dental
composite formulations to their degradation and the release of hydrolyzed polymeric-resin-derived
products. Crit Rev Oral Biol Med. 2001; 12:136-51. 46.
Palmer D, Barco T, Billy E. Temperature extremes produced
orally by hot and cold liquids. J Prosthet
Dent. 1992; 67:325-27. 47.
Turssi C, Hara A, Serra M, Junior A. Effect Of Storage Media
Upon The Surface Micromorphology Or Resin-Based Restorative Materials.
J Oral Rehab. 2002;29:864-71. 48. Urban V, Machado A, Vergani C, Giampaolo E, Pavarina A, de Almeida F, et al. Effect of water-bath
post-polymerization on the mechanical properties,
degree of conversion, and leaching of residual compounds of
hard chairside reline resins. Dent
Mater. 2009;25:662-71. 49.
Urban V, Machado A, Alves M, Maciel
A, Vergani C, Leite E. Glass transition temperature of hard chairside reline materials after
post-polymerisation treatments. Gerodontology. 2010;27:230-5. 50.
Colebeck A, Monaco E, Pusateri
C, Davis E. Microtensile Bond Strength of Different Acrylic Teeth to
High-Impact Denture Base Resins. J Prosthodont Res.
2014;24: 43-51. 51.
Minami H, Suzuki S, Minesaki Y, Kurashige
H, Tanaka T. In vitro evaluation of the influence of repairing condition of denture
base resin on the bonding of autopolymerizing resins.
J Prosthet
Dent. 2004; 91:164-70. 52.
Neves CB, Costa J, Nepomuceno
L, Madeira A, Portugal J, Bettencourt A. Microhardness
and flexural strength of chlorhexidine delivery systems
based on acrylic resin. Rev Port Estomatol
Med Dent Cir Maxilofac. 2019;60:104-10. � Cristina Bettencourt Neves. E-mail address: cristina.neves@fmd.ulisboa.pt � Acknowledgments The authors would like to thank VOCO GmbH for
providing the Ufi Gel Hard evaluated in this study. � Ethical disclosures Protection of human and animal subjects. The
authors declare that no experiments were performed on humans or animals for
this study. Confidentiality of data. The
authors declare that no patient data appear in this article. Right to privacy and informed consent. The
authors declare that no patient data appear in this article. � Conflict of interest The authors have no conflicts of interest to declare. � Article history: Received 13 August 2019 Accepted 5 November 2019 Available online 20 December 2019
