
Revista Portuguesa de Estomatologia, Medicina Dentária e Cirurgia Maxilofacial
SPEMD - Rev Port Estomatol Med Dent Cir Maxilofac | 2019 | 60 (2) | 51-58
Original research
Effect of saliva decontamination protocol on the adhesion to dentin with a universal adhesive
Efeito do protocolo de descontaminação salivar na adesão à dentina com um adesivo universal
a Department of Biomaterials, Faculdade de Medicina Dentária, Universidade de Lisboa, Lisbon, Portugal
Ana Filipa Chasqueira - filipach@gmail.com
Article Info
Rev Port Estomatol Med Dent Cir Maxilofac
Volume - 60
Issue - 2
Original research
Pages - 51-58
Go to Volume
Article History
Received on 31/05/2019
Accepted on 10/09/2019
Available Online on 19/09/2019
Keywords
Original research
�
Effect of saliva decontamination protocol on the adhesion to dentin with a universal adhesive
Efeito do protocolo de descontamina��o salivar na ades�o � dentina com um adesivo universal
�
Ana Filipa Chasqueira*, Joana Lu�s, Jaime Portugal
Department of Biomaterials, Faculdade de Medicina Dent�ria, Universidade de Lisboa, Lisbon, Portugal
�
�
http://doi.org/10.24873/j.rpemd.2019.09.452
�
Abstract
Objective: To evaluate the influence and effectiveness of the salivary decontamination protocol after polymerization of a universal adhesive on dentin adhesive strength, after 24 hours and 6 months of ageing.
Methods: Fifty intact molars were sectioned in order to obtain two slices of dentin from each tooth. In all specimens except the control group (C � no contamination), after applying and light-curing the adhesive system (Scotchbond Universal), the adhesion surface was contaminated with human saliva, and was then subjected to a decontamination method that differed between groups (W � decontamination with water; W+A � decontamination with water and reapplication of adhesive; E � decontamination with ethanol; E+A � decontamination with ethanol and reapplication of adhesive). Shear bond strength was tested at 24 hours and 6 months of aging, until fracture, and the failure mode was observed. Data were statistically analyzed using non-parametric Mann-Whitney and Kruskal-Wallis tests (α=0.05).
Results: At 24 hours of aging, the decontaminant (p=0.289) and the reapplication of the adhesive (p=0.072) did not influence the adhesive strength values, and all contaminated groups obtained significantly (p<0.05) lower adhesive strength values than the control group. At 6 months, the reapplication of the adhesive (W+A and E+A) provided increased adhesion values (p=0.001), but no differences were observed between the decontaminants (p=0.314). Only the W+A group yielded a statistically (p=0.376) similar value to the control group.
Conclusion: When there is salivary contamination of the adhesion area after polymerization of the universal adhesive tested, water decontamination should be performed followed by a reapplication of the adhesive.
Keywords: Decontamination, Dentin bonding, Saliva contamination, Shear bond strength, Universal adhesive
�
Resumo
Objetivo: Avaliar a influ�ncia e efic�cia do protocolo de descontamina��o salivar ap�s polimeriza��o de um adesivo universal na resist�ncia adesiva a dentina, ap�s 24 horas e 6 meses de envelhecimento.
M�todos: Cinquenta molares �ntegros foram seccionados, de forma a obter duas fatias de dentina por dente. Com excep��o do grupo controlo (C � sem contamina��o), ap�s aplica��o e polimeriza��o do sistema adesivo (Scotchbond Universal), foi realizada contamina��o da superf�cie de ades�o com saliva humana seguida de descontamina��o, de acordo com o grupo experimental [W � descontamina��o com �gua, W+A � descontamina��o com �gua seguida de reaplica��o do adesivo, E � descontamina��o com etanol, E+A � descontamina��o com etanol seguida de reaplica��o do adesivo]. Foram realizados testes de resist�ncia adesiva a tens�es de corte, as 24 horas e aos 6 meses, at� a fratura, e o tipo de falha de uni�o foi observado. Os resultados obtidos foram sujeitos a testes n�o-param�tricos de Mann-Whitney e Kruskal-Wallis (α=0,05).
Resultados: As 24 horas, o descontaminante (p=0,289) e a reaplica��o do adesivo (p=0,072) n�o influenciaram os valores de resist�ncia adesiva, e todos os grupos com contamina��o obtiveram valores de resist�ncia adesiva inferiores (p<0,05) ao grupo controlo. Aos 6 meses, a reaplica��o do adesivo (W+A e E+A) permitiu aumentar os valores de ades�o (p=0,001) e n�o se observaram diferen�as entre os descontaminantes (p=0,314). Apenas o grupo W+A permitiu obter valor estatisticamente semelhantes (p=0,376) ao grupo controlo.
Conclus�o: Quando existe contamina��o salivar da �rea de ades�o ap�s a polimeriza��o do adesivo universal testado, devera ser realizada descontamina��o com �gua seguida de reaplica��o do adesivo.
Palavras-chave: Descontamina��o, Ades�o a dentina, Contamina��o salivar, Resist�ncia adesiva ao corte, Adesivo universal
�
Introduction
Since the introduction of adhesive techniques in dentistry, one of the most frequent concerns has been to obtain a completely dry field, so that the adhesive procedures are performed in ideal conditions. The salivary contamination during the adhesive protocol leads to increased microleakage and decreased bond strength, causing reduced longevity of composite resin restorations.1, 3 One way to minimize the risk of saliva contamination is reducing the clinical steps of adhesive systems, such as self-etch systems.4
The current adhesive systems are classified based on the number of clinical steps and the way they interact with the substrate, namely with the smear layer. Thus, there are threestep and two-step etch-and-rinse systems, which include a previous application of phosphoric acid and remove the smear layer; and two-step and one-step self-etch systems, which only modify the permeability of the smear layer.5, 6 In the last decade, universal adhesive systems have been developed, giving great versatility and freedom of choice for the clinician.7 Universal adhesives can be used according to the etch-and-rinse, self-etch or selective etch strategies and in several substrates, such as enamel, dentin, ceramic or metal.8
In these systems, functional monomers of 10-methacryloxydecyl dihydrogen phosphate (10-MDP) that allow chemical bonding to the calcium of hydroxyapatite are generally used, making it possible to create a stable adhesive interface over time.9, 10 Simplified adhesive systems, including universal ones, allow an easier handling protocol, with fewer steps and a less sensitive procedure.11
Since the adhesion to dentin is very complex, the literature is quite controversial regarding the effect of contamination on this substrate.11, 12 The decrease in bond strength after contamination has been correlated to the type of adhesive system, to the stage of the procedure in which the contamination occurs and to the type of contaminant, i.e., blood or saliva.13 The literature agrees that blood contamination results in lower adhesive strength values than salivary contamination. A reason for that difference might be the presence of platelets and fibrinogen capable of forming a film on the surface of the tooth, which compromises the adhesion phenomenon.14, 15
There is no consensus about the best protocol to use in case of contamination during the application of an adhesive system.16, 17 If the contamination occurs before the application of the primer, the saliva will occlude the micro-retentions created by the acid, subsequently interfering with the penetration of the adhesive and resulting in a decreased mechanical retention.18 On the other hand, if contamination occurs before polymerization, solvents such as acetone can be used as decontaminants because of their ability to remove unpolymerized monomers.19 However, if contamination occurs after polymerization of the adhesive, the salivary glycoproteins will attach to the previously polymerized adhesive structure, forming a barrier that prevents the copolymerization of the composite resin, thus affecting the quality of adhesion.20
The main objective of this study is to evaluate the influence of two saliva decontamination protocols on the adhesive strength of a composite resin to dentin at 24 hours and 6 months after the adhesion procedure, according to the following null hypotheses: 1) Saliva contamination followed by decontamination does not influence the bond strength results; 2) The decontamination protocol does not influence the bond strength results.
Materials and Methods
This study was approved by the ethics committee of the Faculty of Dental Medicine, University of Lisbon. The teeth were collected without identification of the donors. They were stored for one week in a 1% chloramine solution at 4 �C and then moved to distilled water until use (ISO / TS 11405/2015).
The sample size (n=10) was estimated with a power analysis based on a pilot study in order to provide statistical significance (=0.05) at 80% power.21 One hundred dentin slices were obtained from 50 human molars and randomly assigned to ten experimental groups to evaluate the influence of the decontamination protocol (decontaminant / adhesive reapplication) on the shear bond strength (SBS), 24 hours and 6 months after the adhesion procedure (Figure 1).
�
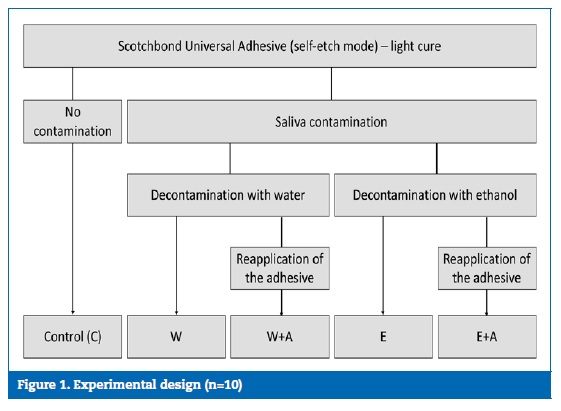
�
Three longitudinal cuts were made in the vestibular-lingual direction of the crowns in order to remove the interproximal enamel and obtain two dentin slices from each tooth. The cuts were made with a diamond precision saw (Isomet� Diamond Wafering Blades, 15 HC, reference 11-4244, Buehler, Illinois, USA) mounted on a microtome (Isomet 1000 precision saw, Buehler, Illinois, USA). A smear layer was created by polishing with a 400-grit silicon carbide sandpaper for 5 seconds under water irrigation.22 The dentin surface was covered with a perforated self-adhesive tape (Glossy White, Xerox, Connecticut, USA) in order to standardize a 3-mm diameter area for adhesion. A universal adhesive (Scotchbond Universal Adhesive, 3M ESPE, Maplewood, MN, USA; Lot 3184625; Val: 2019/06) was actively applied over the dentin, in the self-etch mode, by rubbing it in for 20 seconds, drying it with a gentle airstream for 5 seconds and light-curing it for 10 seconds (Bluephase 20i, Ivoclar Vivadent, Schaan, Liechtenstein; 1250 mW/cm2), following the manufacturer�s instructions.
In all groups, except the control (C), the specimens were then contaminated with saliva for 5 seconds.17 The saliva was collected from a healthy male donor in the morning and before any food intake.23 Subsequently, the following four decontamination protocols were carried out in different experimental groups: W) Decontamination with water and no reapplication of the adhesive, by washing the contaminated surface with running distilled water for 10 seconds and air drying it for 5 seconds; W+A) Decontamination with water followed by reapplication of the adhesive, by washing the contaminated surface with running distilled water for 10 seconds, air drying it for 5 seconds and reapplying the universal adhesive, which was then light-cured; E) Decontamination with ethanol and no reapplication of the adhesive, by actively applying 70% ethanol on the contaminated surface with a microbrush for 10 seconds and drying it for 5 seconds; E+A) Decontamination with ethanol followed by reapplication of the adhesive, by actively applying 70% ethanol on the contaminated surface with a microbrush for 10 seconds, drying it for 5 seconds and reapplying the universal adhesive, which was then light-cured.
After the adhesive protocol, two 2-mm increments of a nanohybrid composite resin (Tetric Evoceram, Ivoclar Vivadent, Schaan, Liechtenstein; Lot V17575; Val 2020/04), shade A3.5, were then applied and light-cured for 20 seconds each.
Half of the specimens of each group were stored in distilled water at 37 �C for 24 hours before testing. The other 50 specimens were immersed in sodium azide solution (pH≈7) at 37 �C and aged for 6 months. The antibacterial solution was changed monthly.
The SBS tests were performed with a single-plane lap device in a universal testing machine (Instron, model 4502, series # H3307 � Instron Ltd, Bucks, England) with a load cell of 1 KN and a crosshead speed of 1 mm/min, until failure. The fracture surface was analysed with a stereomicroscope (Meiji Techno Co., model EMZ-8TR, series No. 41179, Saitama, Japan) under a magnification of 20X. The failure mode was classified as �adhesive� when it occurred at the resin/dentin interface; �cohesive� when it was exclusive to the dentin or composite resin; or �mixed� when it had a combination of the two previous types.24
Data were analyzed using IBM SPSS Statistics, version 21.0 (SPSS Inc., Chicago, Illinois, USA). Since the Shapiro-Wilk and Levene�s tests (p<0.05) revealed no normality and homogeneity, SBS data were analyzed with Mann-Whitney and Kruskal-Wallis non-parametric statistical tests. The failure mode was analyzed statistically using the chi-square test. A statistical significance of α=0.05 was considered for all tests.
Results
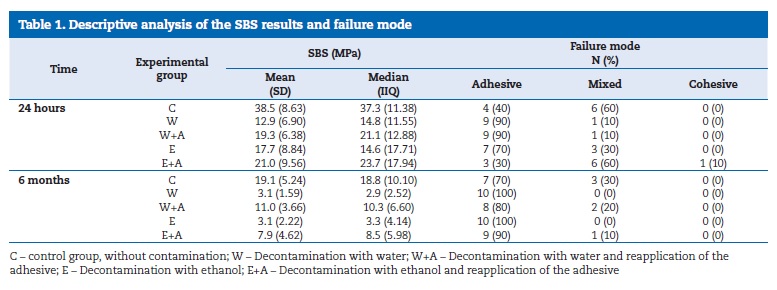
The median SBS values, at 24 hours, ranged from 14.6 MPa, in group E, to 37.3 MPa, in group C (Table 1). At 6 months, the median ranged between 2.9 MPa, in group W, and 18.8 MPa, in group C (Table 1).
�
�
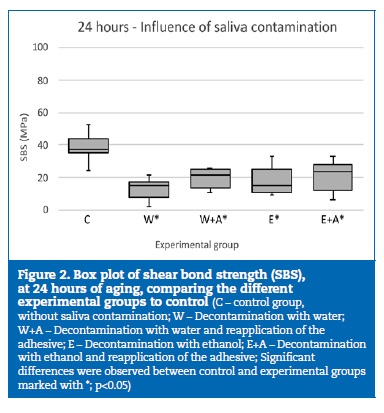
A statistically significant (p<0.001) decrease of the bond strength was observed throughout the aging period. At 24 hours of aging, all the experimental groups where contamination occurred showed a significant (p<0.05) decrease in SBS results, compared to the control group (Figure 2).
�
�
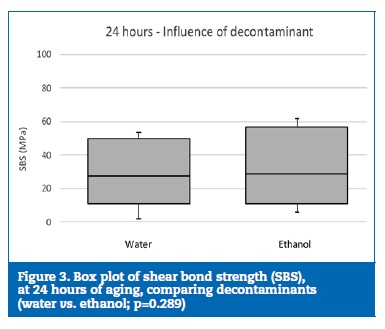
However, no statistically significant (p=0.289) differences were found between the decontaminant agents (Figure 3), and the reapplication of the adhesive did not statistically (p=0.072) influence the bond strength (Figure 4).
�
�
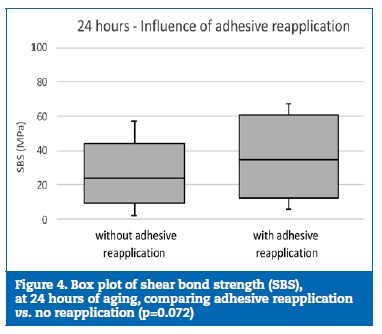
�
At 6 months of aging, only the group W+A yielded similar statistical results to group C (p=0.376) (Figure 5). Although no statistically significant differences (p=0.314) were detected between the decontamination agents (Figure 6), the specimens where the reapplication of the adhesive was performed showed statistically (p<0.001) higher bond strength results than the specimens with no adhesive reapplication (Figure 7).
�
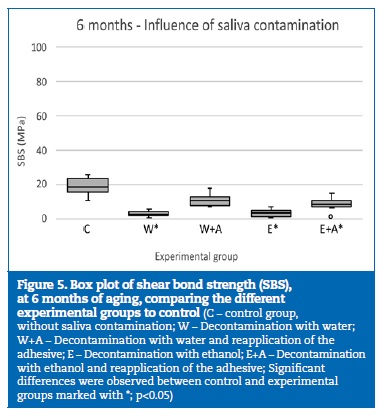
�
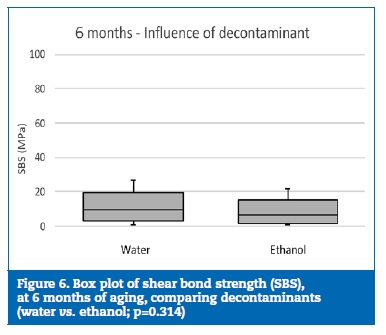
�
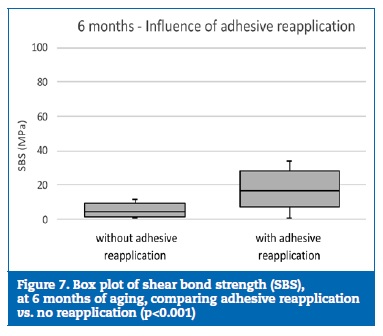
�
The failure mode was predominantly adhesive (65%), and no statistically significant differences (p=0.677) were found.
Discussion
The salivary contamination was responsible for the decrease in the SBS results, at both 24 hours and 6 months of aging.
Thus, the first null hypothesis was rejected. Similar results had been observed in previous studies.12, 15 Regarding the saliva decontamination agent, no differences were identified between water and ethanol either at 24 hours or at 6 months of aging. Also, no differences were detected between experimental groups (excluding the control group) at 24 hours. However, the same tendency was not verified after 6 months of aging, as the reapplication of the adhesive promoted higher SBS values. Thus, the second null hypothesis was also rejected.
Saliva is one of the sources of contamination during restorative treatments. It consists mainly of water, but also has macromolecules, electrolytes and organic particles.25 The water present in the saliva, as well as the salivary glycoproteins, have been pointed out as the main factors for the reduction of the adhesion to dentin.12, 26, 27 In some in vitro studies, artificial saliva is used as a contaminant.28, 29 However, the properties of these solutions are not equal to human saliva and may introduce some errors. In order to simulate the conditions of the oral cavity, human saliva was used in this study.
The SBS assay was chosen because it allows an easy preparation of the specimens and a technically simple and predictable execution.30, 33
Other studies have also shown a decrease in the bond strength of the Scotchbond Universal adhesives over time, especially if applied in the self-etch mode.34 The degradation of the adhesive interface, especially in simplified systems, has been associated with its hydrophilic characteristics that allow a great absorption of water.35, 36 Although there is not much evidence in the literature about the effect of contamination and subsequent decontamination methods on adhesion promoted by universal adhesives, it can be inferred that the presence of saliva accelerates the degradation process of the adhesive interface, because it behaves as semipermeable membranes, even after polymerization.37
According to the manufacturer, the Scotchbond Universal adhesive system is tolerant of small amounts of saliva before the application of the adhesive. This feature may be explained by the several components in its composition that may render it less susceptible to moisture. These components include 10-MDP monomers, which decrease the susceptibility to hydrolysis; an optimized amount of hydrophilic 2-hydroxyethyl methacrylate (HEMA) monomers, which promote adhesion to wet substrates; and a Vitrebond� copolymer, which has been associated with increased bond strength on wet substrates in several adhesives.38 40
One-bottle adhesives have been considered to be more resistant to moisture due to their hydrophilic properties and their acidic capacity that allows the breakdown of saliva mucopolysaccharides.39 Also, they generally include solvents such as ethanol or acetone, capable of denaturing salivary glycoproteins, thus reducing the negative effect of contamination.41
However, this higher tolerance seems to be dependent on the phase of the adhesive procedure in which the contamination occurs.2, 42 The decrease in bond strength has been explained by the physical barrier caused by the impregnation of salivary glycoproteins in the adhesive layer, which is poorly polymerized due to inhibition by oxygen.8, 43, 44 Thus, the presence of macromolecules may prevent the infiltration of the monomers into the collagen network when contamination occurs before the application of the adhesive or lead to a reduced wetting of the composite resin when it occurs after polymerization of the adhesive.17 In addition, the water incorporated in this newly polymerized layer of adhesive may interfere with the copolymerization of the subsequent composite resin.45
Since ethanol can promote a greater dispersion of the water and has disinfectant properties, it could be advantageous for the decontamination of a surface contaminated with saliva, promoting superior adhesive forces. However, this influence was not verified in this study, since no differences were found between saliva decontamination with water or ethanol, either at 24 hours or at 6 months of aging.
Regarding the reapplication of the adhesive, no differences between reapplying it or not were found at 24 hours. However, after 6 months of aging, the groups where the adhesive was reapplied showed higher bond strength than those in which it was not; even so, this did not allow to re-establish bond strength, as has been reported.1, 46, 47 The reapplication of the adhesive increases the adhesive thickness and promotes a lower hydrolytic degradation potential over time.37
Reapplying the adhesive will probably provide a new layer of free bonding monomers with a capacity of chemical bonding to the above restorative resin, thus increasing the quality of the adhesion.
Most failures occurred between the adhesive and the composite resin, as expected, since SBS values generally decline after salivary contamination.
Further studies with other adhesive systems should be performed in the future to verify if they also show an advantage of reapplying the adhesive under salivary contamination conditions. Also, another type of assay, such as nanoleakage, may be performed to evaluate the permeability of the adhesive interface under the studied conditions.
Conclusion
In the conditions of the present study, the contamination with saliva, after light-curing the Scotchbond Universal Adhesive, caused a decreased bond strength of the composite resin to dentin at 24 hours, regardless of the decontamination protocol used. However, saliva decontamination with water followed by adhesive reapplication yielded similar bond strength values than non-contaminated specimens, at 6 months of aging.
�
References
1. Park JW, Lee KC. The influence of salivary contamination on shear bond strength of dentin adhesive systems. Oper Dent. 2004;29:437-42.
2. Yoo HM, Oh TS, Pereira PN. Effect of saliva contamination on the microshear bond strength of one-step self-etching adhesive systems to dentin. Oper Dent. 2006;31:127-34.
3. Yoo HM, Pereira PN. Effect of blood contamination with 1-step self-etching adhesives on microtensile bond strength to dentin. Oper Dent. 2006;31:660-5.
4. Brauchli L, Eichenberger M, Steineck M, Wichelhaus A. Influence of decontamination procedures on shear forces after contamination with blood or saliva. Am J Orthod Dentofacial Orthop. 2010;138:435-41.
5. Van Meerbeek B, De Munck J, Yoshida Y, Inoue S, Vargas M, Vijay P, el al. Buonocore memorial lecture. Adhesion to enamel and dentin: current status and future challenges. Oper Dent. 2003;28:215-35.
6. Pashley DH, Tay FR, Breschi L, Tjaderhane L, Carvalho RM, Carrilho M, et al. State of the art etch-and-rinse adhesives. Dent Mater. 2011;27:1-16.
7. Munoz M, Luque-Martinez I, Malaquias P, Hass V, Reis A, Campanha N, et al. In vitro longevity of bonding properties of universal adhesives to dentin. Oper Dent. 2015;40:282-92.
8. Perdigao J, Kose C, Mena-Serrano AP, De Paula EA, Tay LY, Reis A, et al. A new universal simplified adhesive: 18-month clinical evaluation. Oper Dent. 2014;39:113-27.
9. Yoshida Y, Yoshihara K, Nagaoka N, Hayakawa S, Torii Y, Ogawa T, et al. Self-assembled nano-layering at the adhesive interface. J Dent Res. 2012;91:376-81.
10. Osorio R, Toledano M, de Leonardi G, Tay F. Microleakage and interfacial morphology of self-etching adhesives in class V resin composite restorations. J Biomed Mater Res B Appl Biomater. 2003;66:399-409.
11. Taskonak B, Sertgoz A. Shear bond strengths of saliva contaminated �one-bottle� adhesives. J Oral Rehabil. 2002;29:559-64.
12. Fritz UB, Finger WJ, Stean H. Salivary contamination during bonding procedures with a one-bottle adhesive system. Quintessence Int. 1998;29:567-72.
13. Kim J, Hong S, Choi Y, Park S. The effect of saliva decontamination procedures on dentin bond strength after universal adhesive curing. Restor Dent Endod. 2015;40:299-305.
14. Prasad M, Mohamed S, Nayak K, Shetty SK, Talapaneni AK. Effect of moisture, saliva and blood contamination on the shear bond strength of brackets bonded with a conventional bonding system and self-etched bonding system. J Nat Sci Biol Med. 2014;5:123-9.
15. Koppolu M, Gogala D, Mathew VB, Thangala V, Deepthi M, Sasidhar N. Effect of saliva and blood contamination on the bond strength of self-etching adhesive system- An in vitro study. J Conserv Dent. 2012;15:-270-3.
16. Silverstone LM, Hicks MJ, Featherstone MJ. Oral fluid contamination of etched enamel surfaces: an SEM study. J Am Dent Assoc. 1985;110:329-32.
17. Neelagiri K, Kundabala M, Shashi RA, Thomas MS, Parolia A. Effects of saliva contamination and decontamination procedures on shear bond strength of self-etch dentine bonding systems: An in vitro study. J Conserv Dent. 2010;13:71-5.
18. Khanehmasjedi M, Naseri MA, Khanehmasjedi S, Basir L. Comparative evaluation of shear bond strength of metallic brackets bonded with two different bonding agents under dry conditions and with saliva contamination. J Chi Med Assoc. 2017;80:103-8.
19. Elkassas D, Arafa A. Assessment of post-contamination treatments affecting different bonding stages to dentin. Eur J Dent. 2016;10:327-32.
20. Sattabanasuk V, Shimada Y, Tagami J. Effects of saliva contamination on dentin bond strength using all-in-one adhesives. J Adhes Dent. 2006;8:311-8.
21. Rosca B, Ramalho S, Sampaio-Fernandes JC, Portugal J. Reparability of two different CAD/CAM polymer materials using light-cured composite and universal adhesives. Ver Port Estomatol Med Dent Cir Maxilofac. 2016;57:189-96.
22. Oliveira SS, Pugach MK, Hilton JF, Watanabe LG, Marshall SJ, Marshall GW Jr. The influence of the dentin smear layer on adhesion: A self-etching primer vs. a total-etch system. Dent Mater. 2003;19:758-67.
23. Pitta J, Branco TC, Portugal J. Effect of saliva contamination and artificial aging on different primer/cement systems bonded to zirconia. J Prosthet Dent. 2018;119:833-9.
24. Luque-Martinez IV, Perdigao J, Munoz MA, Sezinando A, Reis A, Loguercio AD. Effects of solvent evaporation time on immediate adhesive properties of universal adhesives to dentin. Dent Mater. 2014;30:1126-35.
25. Humphrey SP, Williamson RT. A review of saliva: Normal composition, flow, and function. J Prosthet Dent. 2001; 85:162-9.
26. Xie J, Powers JM, McGuckin RS. In vitro bond strength of two adhesives to enamel and dentin under normal and contaminated conditions. Dent Mater. 1993;9:295-9.
27. el-Kalla IH, Garcia-Godoy F. Saliva contamination and bond strength of single-bottle adhesives to enamel and dentin. Am J Dent. 1997;10:83-7.
28. Darabi F, Tavangar M, Davalloo R. Effect of different decontamination procedures from a saliva-contaminated cured bonding system (Single Bond). Dent Res J (Isfhan).2012;9:399-403.
29. Gal JY, Fovet Y, Adib-Yadzi M. About a synthetic saliva for in vitro studies. Talanta. 2001;53:1103-15.
30. Braga RR, Meira JB, Boaro LC, Xavier TA. Adhesion to tooth structure: A critical review of �macro� test methods. Dent Mater. 2010;26:e38-49.
31. Placido E, Meira JB, Lima RG, Muench A, de Souza RM, Ballester RY. Shear versus micro-shear bond strength test: A finite element stress analysis. Dent Mater. 2007; 23:1086-92.
32. Chasqueira F, Portugal J, Arantes SSA, Lopes LP. Adhesion of dental sealants to enamel with self-etching adhesives in salivary contamination conditions: Influence of the light curing protocol. Rev Port Estomatol Med Dent Cir Maxilofac. 2011;52:2-6.
33. Chasqueira AF, Arantes-Oliveira S, Portugal J. Effect of changes to the manufacturer application techniques on the shear bond strength of simplified dental adhesives. J Appl Biomater Funct Mater. 2013;11:e117-21.
34. Marchesi G, Frassetto, A, Mazzoni A, Apolonio F, Diolosa M, Cadenaro M, et al. Adhesive performance of a multi-mode adhesive system: 1-Year in vitro study. J Dent. 2014;42:603-12.
35. Ito S, Hashimoto M, Wadgaonkar B, Svizero N, Carvalho RM, Yiu C, et al. Effects of resin hydrophilicity on water sorption and changes in modulus of elasticity. Biomaterials.2005;26:6449-59.
36. Nishitani Y, Yoshiyama M, Donnelly AM, Agee KA, Sword J, Tay FR, et al. Effects of resin hydrophilicity on dentin bond strength. J Dent Res. 2006;85:1016-21.
37. Tay FR, Frankenberger R, Krejci I, Bouillaguet S, Pashley DH, Carvalho, RM, et al. Single-bottle adhesives behave as permeable membranes after polymerization. I. In vivo evidence. J Dent. 2004;32:611-21.
38. Van Landuyt KL, Snauwaert J, De Munck J, Peumans M, Yoshida Y, Poitevin A, at al. Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials. 2007;28:3757-85.
39. Sheikh H, Heymann HO, Swift EJ Jr, Ziemiecki TL, Ritter AV. Effect of saliva contamination and cleansing solutions on the bond strengths of self-etch adhesives to dentin. J Esthet Restor Dent. 2010;22:402-10.
40. Furuse AY, Cunha LF, Moresca R, Paganeli G, Mondelli RF, Mondelli J. Enamel wetness effects on bond strength using different adhesive systems. Oper Dent. 2011;36:274-80.
41. Eirksson SO, Pereira PN, Swift EJ Jr, Heymann HO, Sigurdsson A. Effects of saliva contamination on resin-resin bond strength. Dent Mater. 2004;20:37-44.
42. Santschi K, Peutzfeldt A, Lussi A, Flury S. Effect of salivary contamination and decontamination on bond strength of two one-step self-etching adhesives to dentin of primary and permanent teeth. J Adhes Dent. 2015;17:51-7.
43. Hiraishi N, Kitasako Y, Nikaido T, Nomura S, Burrow MF, Tagami J. Effect of artificial saliva contamination on pH value change and dentin bond strength. Dent Mater. 2003;19:429-34.
44. Kermanshah H, Ghabraei S, Bitaraf T. Effect of salivary contamination during different bonding stages on shear dentin bond strength of one-step self-etch and total etch adhesive. J Dent (Tehran). 2010;7:132-8.
45. Abdalla AI, Davidson CL. Bonding efficiency and interfacial morphology of one-bottle adhesives to contaminated dentin surfaces. Am J Dent. 1998;11:281-5.
46. Ari H, Donmez N, Belli S. Effect of artificial saliva contamination on bond strength to pulp chamber dentin. Eur J Dent. 2008;2:86-90.
47. Patil SB, Shivakumar AT, Shah S. Effect of salivary contamination on shear bond strength os two adhesives: An in vitro study. Dent Hypotheses. 2014;5:115-20.
�
Ana Filipa Chasqueira
E-mail address: filipach@gmail.com
�
Ethical disclosures
Protection of human and animal subjects. The authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of data. The authors declare that no patient data appear in this article.
Right to privacy and informed consent. The authors declare that no patient data appear in this article.
�
Conflict of interest
The authors have no conflicts of interest to declare.
�
Article history:
Received 31 May 2019
Accepted 10 September 2019
Available online 19 September 2019
