
Revista Portuguesa de Estomatologia, Medicina Dentária e Cirurgia Maxilofacial
SPEMD - Rev Port Estomatol Med Dent Cir Maxilofac | 2019 | 60 (1) | 1-7
Original research
Corrosion resistance and surface characterization of miniscrews removed from orthodontic patients
Resistência à corrosão e caraterização da superfície de mini-implantes removidos de pacientes ortodonticos
a Universidade Federal de Juiz de Fora, Juiz de Fora, Minas Gerais, Brasil
b Instituto Federal do Sudeste de Minas Gerais, Juiz de Fora, Minas Gerais, Brasil
Marcelo Santos Bahia - marcelosbahia@outlook.com
Article Info
Rev Port Estomatol Med Dent Cir Maxilofac
Volume - 60
Issue - 1
Original research
Pages - 1-7
Go to Volume
Article History
Received on 12/02/2019
Accepted on 13/05/2019
Available Online on 29/05/2019
Keywords
Original research
�
Corrosion resistance and surface characterization of removed from orthodontic patients
Resist�ncia � corros�o e carateriza��o da superf�cie de mini-implantes removidos de pacientes ortodonticos
�
Paula Liparini Caetanoa, Marcelo Santos Bahiaa,*, Elison da Fonseca e Silvab, Robert Willer Farinazzo Vitrala, Marcio Jos� da Silva Camposa
a Universidade Federal de Juiz de Fora, Juiz de Fora, Minas Gerais, Brasil
b Instituto Federal do Sudeste de Minas Gerais, Juiz de Fora, Minas Gerais, Brasil
�
�
http://doi.org/10.24873/j.rpemd.2019.05.445
�
�
Abstract
Objectives: To assess the corrosion resistance and the surface microstructure of orthodontic mini-implants removed after being in function in their bone insertion sites.
Methods: Twenty orthodontic mini-implants made of Ti6AI4V alloy were assessed, divided into two groups of 10 units: the control group and the test group. The test group was composed of implants that were removed after being stable in function in their bone insertion sites for 230 days). The visual analysis of the thread surface of mini-implants was performed with a scanning electron microscope (SEM) and the evaluation of corrosion resistance with the cyclic potentiodynamic polarisation test.
Results: No significant differences were identified between the groups, although the comparison between the OCP (open circuit potential) values had a p value =0.050. The SEM images of the thread surface of the mini-implants of both groups showed a regular polished surface. Only one mini-implant from the control group showed the presence of pitting from the corrosion process.
Conclusions: The average duration of 230 days in function of the orthodontic mini-implants made of Ti6Al4V alloy did not cause significant changes in corrosion resistance nor in the superficial characteristics of these devices
Keywords: Corrosion, Orthodontic anchorage procedures, Orthodontics, Scanning electron microscope
�
Resumo
Objetivos: Avaliar a resist�ncia � corros�o e a microestrutura superficial de mini-implantes ortod�nticos mantidos em seus locais de inser��o �ssea e removidos ap�s o uso.
M�todos: Foram avaliados 20 mini-implantes ortod�nticos de liga de Ti6AI4V; divididos em 2 grupos de 10 unidades: o grupo controle e o grupo teste (que permaneceu est�vel em seus locais de inser��o �ssea e que foram removidos ap�s o uso) com dura��o de 230 dias. A an�lise visual da superf�cie da rosca dos mini-implantes foi realizada com microsc�pio eletr�nico de varredura (MEV) e a avalia��o da resist�ncia � corros�o com o teste de polariza��o potenciodin�mica c�clica.
Resultados: N�o foi identificada nenhuma diferen�a significativa entre os grupos, embora a compara��o entre os valores de OCP (potencial de circuito aberto) tenha p-valor = 0,050. Imagens de MEV da superf�cie da rosca dos mini-implantes obtidos de ambos os grupos mostraram uma superf�cie polida regular. Apenas um mini-implante do grupo de controle mostrou a presen�a de corros�o do processo de corros�o.
Conclus�es: A perman�ncia m�dia de 230 dias de mini-implantes ortod�nticos feitos de liga Ti6Al4V em locais de inser��o �ssea n�o causou altera��es significativas nem na resist�ncia � corros�o nem nas caracter�sticas superficiais desses dispositivos.
Palavras-chave: Corros�o, Procedimentos de ancoragem ortod�ntica, Ortodontia, Microsc�pio eletr�nico de varredura
�
Introduction
The orthodontic anchorage can be defined as the resistance to an unwanted tooth movement in order to maximise the desired movements.1 In that sense, the mini-implants have emerged as an alternative mean to control the anchorage,1 while allowing teeth to be moved in the three spatial planes with reduced unwanted movements.2 3
Commercially pure titanium (Ti cp), the titanium-aluminium- vanadium alloy (Ti6Al4V) and surgical stainless steel have been used as raw materials for some biomaterials, as in the case of orthodontic mini-implants.4 - 8 Studies have compared these materials and concluded that the Ti6Al4V alloy offers advantages over surgical stainless steel because of its improved biocompatibility, high bacteriostatic action and high resistance to corrosion, which is attributed to the formation of a protective layer of titanium dioxide (TiO2).5, 8 - 11 Compared to Ti cp, the Ti6Al4V alloy offers the advantage of having greater mechanical resistance, but is less corrosion-resistant. 5, 9 - 12
In general, biomaterials must present some specific features, such as biocompatibility and corrosion resistance, to be used in the human organism.13, 15 It is essential to know the type and amount of substances released by the material and the reaction of organic tissues to them.16, 18 The insertion of devices in the human body can cause adverse effects on the tissues due to the release of metal ions originated from material corrosion. 16, 19 The orthodontic mini-implants must have, in addition to specific mechanical resistance, corrosion resistance in the physiological environment in which they will be inserted.18,20 Therefore, corrosion resistance is one of the main points to investigate in order to determine the biocompatibility of these devices.18
This study aims to understand the interaction of the mini-implant with the surrounding bone tissues in situations in which this anchorage device is clinically used, by assessing the corrosion resistance and surface microstructure of orthodontic mini-implants that were removed after being in function in their bone insertion sites. The following null hypotheses were tested: (1) the duration of mini-implants in function does not influence their resistance to corrosion; (2) the duration of mini-implants in function does not alter their surface microstructure.
Materials and methods
The sample was composed of twenty 6-mm-long and 1.5-mm-diameter orthodontic mini-screw implants, manufactured with Ti6AI4V alloy (titanium-aluminium-vanadium alloy), all from the same batch. The mini-implants were divided into two groups of 10 units each:
� Group 1: mini-implants in their original form, as received from the manufacturer;
� Group 2: mini-implants that were removed from orthodontic patients after being stable in function.
- Group 2 mini-implants were used in various orthodontic mechanics and hade been inserted on average for 230 days (7.66 months [SD 64.807]). The procedures of insertion and removal of the mini-implants were performed manually by the same qualified and trained operator.
This study was approved by the Ethics Committee of the Federal University of Juiz de Fora. After removal, the mini-implants were placed in individual glass containers and cleaned in an ultrasonic tank (LS-0, 8D LimpSonic, S�o Paulo, Brazil) with enzymatic detergent for 30 minutes and with acetone for 30 minutes to remove organic materials and oily residues from their surface. After cleaning, the mini-implants were placed in individual glass containers and stored in a desiccator in order to control the humidity of the microenvironment and stabilise the corrosion process.
For the corrosion resistance evaluation, all mini-implants were subjected to a cyclic potentiodynamic polarisation test in a PGSTAT 204N potentiostat (MetrohmAutolab BV, Utrecht, Netherlands) controlled by NOVA 2.0 (MetrohmAutolab BV, Utrecht, Netherlands). The corrosion resistance of mini-implants was determined by the potential for pitting formation, as identified in the anodic polarisation curve.
For the polarisation testing, we used the three-electrode scheme with the electrodes immersed in a working solution.
The working electrode was composed of the mini-implant with a 1-mm-thick laminated tip of copper wire attached to its head.
To standardise the area of the working electrode exposed to the working solution, a negative model of the mini-implant was Made in addition silicone (Zhermack, Italy). The body of the mini-implant (thread region of 6 mm) was inserted in the negative model, and only the head of the mini-implant with the copper wire attached was left free and these were both isolated with resin-based thermoplastic and rubber adhesive. A platinum large-area electrode was used as a counter electrode and served as a cathode receiving electrons released by the mini-implant. A third Ag/AgCl electrode served as the reference electrode. The distance kept between the electrodes in the electrochemical cell was standardised in all experiments.
The electrodes were immersed in 50 mL of Ringer�s lactate solution (changed between experiments) kept at 37�C �1�C by a copper coil system with circulating heated water (Ultra-thermostatic Bath SL 152, Solab, Piracicaba, Brazil). Every 100 mL of Ringer�s lactate solution was composed of 0.3 g of sodium lactate, 0.6 g of sodium chloride, 0.03 g of potassium chloride and 0.02 g of calcium chloride. This solution was chosen due to its electrochemical characteristics and its isotonicity to blood plasma.
Before the experiment, the electrodes were kept in the solution to reach electrochemical equilibrium and establish the value of the open circuit potential (OCP). The time that each mini-implant took to achieve this equilibrium was described as tOCP. The polarisation test started from the OCP value, increased at a rate of 0.33 mV/s (1.2 V/h) and the final potential value was stipulated at 1.4 V above the OCP. This value of 1.4 V is the maximum limit of the experiment; from this potential value on, it becomes extremely difficult to distinguish between the current originating from the corrosion of the material studied and the one originating from the water dissociation reaction.20
With the polarisation test, OCP, ipp (primary passivation current � current endpoint in the stretch of passivation) and Epp (primary passivation potential � potential endpoint in the stretch of passivation) measures were obtained.
The entire experiment was conducted inside a Faraday cage, with the goal of isolating the system from external electromagnetic waves, thus avoiding interference and favouring a more reliable result. After the polarisation test, the procedures for cleaning and storing the mini-implants were carried out.
The mini-implants� surface microstructure was assessed visually, using a scanning electron microscope (LEICA/LEO Stereoscan S440-EMU, Rondebosch, South Africa) equipped with a retro-spread electron detector and secondary electrons, at 100x to 500x magnification. When any suggestive image of pitting was found, the energy dispersive x-ray spectrometry (EDX), which provides a chemical characterisation of the studied material, was carried out.
The distribution pattern of the tOCP, OCP, ipp and Epp values was evaluated by the Shapiro-Wilk test. The Student t-test for independent samples was used to compare the two groups (1 and 2). The statistical analysis considered a level of significance of α=0.05, and data were processed with the SPSS Statistics 20.0.0 software (SPSS, Chicago, IL, USA).
Results
The tOCP, OCP, Epp and ipp variables showed normal distribution in both groups (Table 1), indicating the use of parametric tests.�
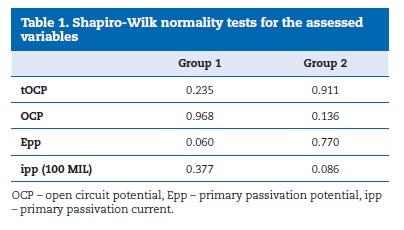
�
Descriptive data regarding the tOCP, OCP, Epp and ipp variables of mini-implants from groups 1 and 2 are shown in Table 2.
�
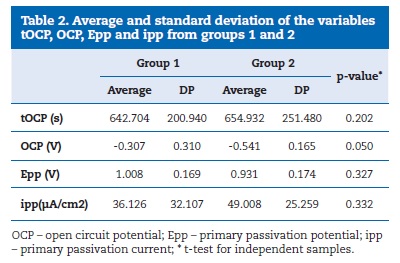
�
No significant differences were identified between the groups for the studied variables, although the comparison between the OCP values (the stabilisation potential was checked within approximately 11 minutes in both groups) had a threshold p value (p=0.050). The power of statistical significance was 0.699, which corresponds to a 70% chance of having a real effect. The statistical power was calculated based on the Epp variable, which would, hypothetically, show the biggest difference between the groups if the mini-implants removed from patients presented less resistance to corrosion. In general, the polarisation curves obtained for the mini-implants from groups 1 (Figure 1) and 2 (Figure 2) showed no indications of pitting corrosion. The only exception was mini-implant number 3 from Group 1 (Figure 3), which showed a sudden current increase measured in the potential of 0.80 V, characterised by a horizontal plateau in the chart, thus suggesting a pitting corrosion process.�
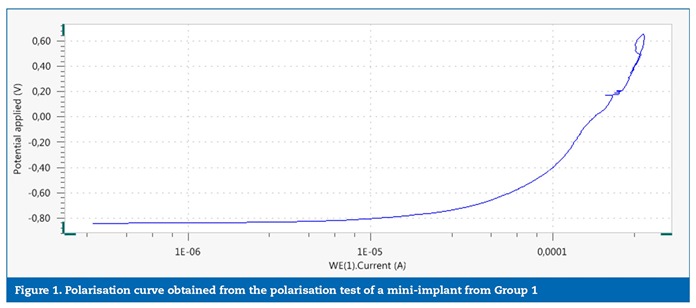
�
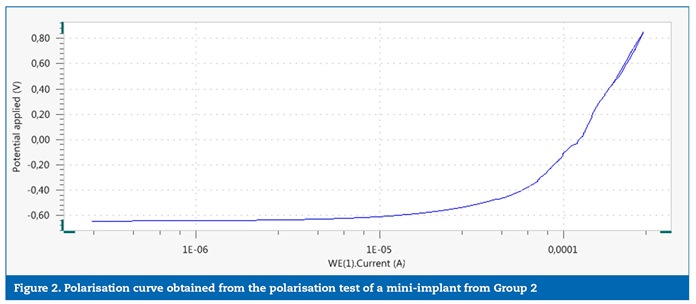
�
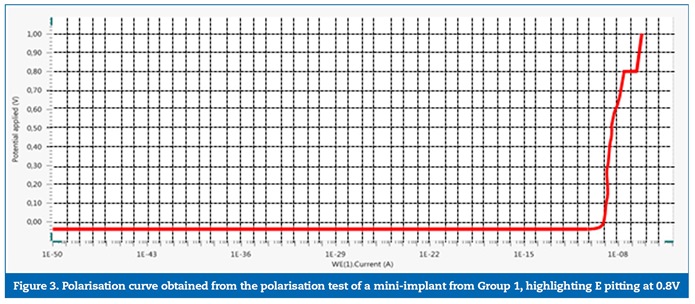
�
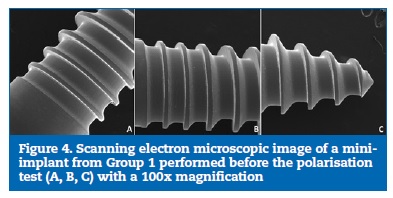
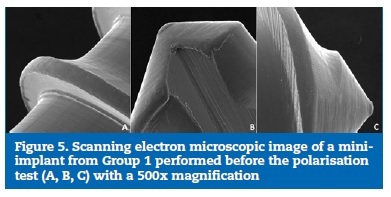
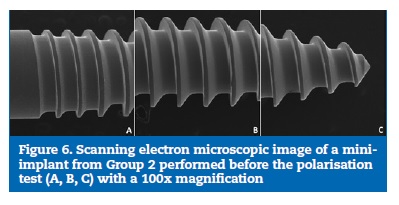
The SEM images of the thread surface of the mini-implants from both Group 1 (Figures 4 and 5) and Group 2 (Figures 6 and 7) obtained before the cyclic polarisation test showed a polished and regular surface with no stains or adhered particles.
�
�
�
�
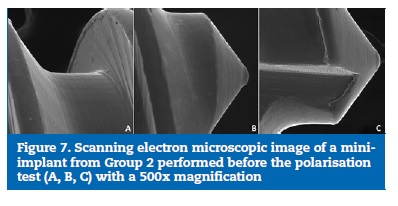
�
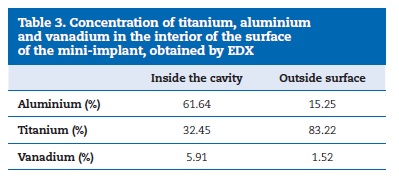
After the polarisation test, the assessment with SEM was performed only for mini-implant number 3 from Group 1 and revealed a cavity suggesting pitting corrosion (Figure 8). An EDX analysis was carried out on that cavity and showed a 61%reduction in the concentration of titanium within the cavity compared to 15% on the surface of the mini-implant (Table 3), thus confirming the presence of pitting from a corrosion process.
�
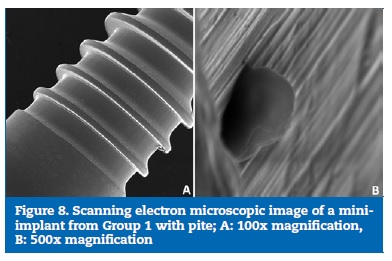
�
Discussion
One of the parameters to determine the biocompatibility of a material is its corrosion resistance after insertion in the body, and the potentiodynamic polarisation test is one of the most widely used methods to test it.21 Potentiodynamic polarization can be performed with two21,22 or three electrodes, 5, 6, 23 - 26 as performed in the present research. In the system of three electrodes, the current is directed from the working electrode to the counter electrode while the potential is measured between the working and reference electrodes. The use of this system has provided more accurate measurements because the absence of an electron current in the reference electrode kept the potential constant, therefore providing a stable reference for the measurements of the test.27
The material used in the reference electrode and the counter electrode also influences the polarisation test as some materials may dissolve during the test, thus affecting the results.27 In this research and previous studies, 5, 25 platinum and silver electrodes were used, which, along with gold, are the materials indicated for studies on kinetics and mechanisms of electron transfer due to their minimal dissolution.25
As in the present research, previous studies used discs or cylinders made of Ti6Al4V alloy24 or the device in its original form22, 25 as the working electrode to determine the electrochemical behaviour of mini-implants. Using mini-implants allows simulating the energy released by chemical reactions (enthalpy) and the molecular disorganisation according to the contact area (entropy) that occur when the device is in function. 28, 29
Since the object of evaluation of this research was the portion of the mini-implant that remains inserted in the bone tissues of the patient, the solution for the experiment should mimic the electrolytic aggression caused by the environment and, so, Ringer�s lactate solution was chosen as it is isotonic to blood plasma.30, 31 Artificial saliva was described as an environment in polarisation tests. 5, 22, 24, 25 However, choosing this solution would only make sense if the study assessed the corrosion resistance of the head and neck of the mini-implant,24, as then it would simulate its clinical application.
The open circuit potential (OCP) is defined as the potential of an electron conducting material immersed in an electrolytic ion-conducting medium;25 and is obtained when the electrodes in open circuit achieve a relative electrolytic stabilization with little variation of the potential with time; it depends on the environment of the test and the exposure time.25 The potential stabilisation in the present study was seen within approximately 11 minutes in both groups, which is lower than in other studies that reported the potential stabilisation after 20 minutes,5, 60 minutes, 22, 23, 25, 26 120 minutes24 and 250 minutes21 of keeping the electrodes in open circuit.
Although the statistical comparison was on the threshold of the established significance, with p=0.05, Group 2 showed an OCP value 43% lower than Group 1, indicating a higher chemical reactivity and predisposition to corrosion25, of the mini-implants that had been in function. However, no evidence of corrosion was identified on the components of Group 2, which had been inserted in alveolar bone, on average, 230 days. In addition to this, the groups showed no difference in the primary passivation potential (Epp) and in the primary passivation current (ipp), which reflects the equality between the groups with regard to the protective power of the passive layer against corrosion.27 Conversely, in SEM images, Patil et al.32 identified evidence of corrosion in mini-implants that had been in function for 230 days or 390 days, while the control group showed no such superficial changes. However, those authors did not evaluate the electrochemical behaviour of the devices.
Regarding the mini-implant that had a corrosion point (number 3 of Group 1), the sudden increase in the current at the induced potential of 0.80V indicates that, at that time, there was a break in the protective layer and the start of a pitting corrosion process, as confirmed by the EDX result. Immediately after this stage, the rate of current increase returned to the expected level, indicating that there had been repassivation of the protective layer, and the corrosion point was covered by a TiO2 layer.5, At the end of the test, the mini-implant had an Epp of 1.034V � close to the average of its group, but an ipp 98% lower than the average of the group (0.74 μA/cm2), which indicates the fragility of the protection against corrosion from the passive layer.27 Although Patil et al.32 intended to evaluate the behaviour of mini-implants that had been in contact with bone and soft tissue, as well as fluid and food in the oral cavity, and used the MEV and the EDX in their methodology, they did not evaluate the behaviour of the resistance to corrosion, which is done by cyclic polarization tests, as carried out in this research. Using EDX, they evidenced not only the presence of dullness, corrosion and blunting of threads and tips but also the adsorption of several elements of the mini-implants. In the group of mini-implants with imperfections, they observed a high level of iron and cerium in the region of the head and neck compared to the group of new mini-implants (control) and the group of successful mini-implants.
However, independently of its manufacturer and clinical use, it was evidenced that all mini-implants had superficial degradation, plastic deformation and signs of breaking. In this research, after the initial scanning of each mini-implant, some images were captured for detailed analyses of the devices. The visual analyses of images obtained with SEM did not indicate structural imperfections on the surface of the thread of the mini-implants caused by the manufacturing process (Group 1) and by the procedures of insertion and removal of the devices (Group 2), and the mini-implants images were similar in both groups. Conversely, Patil et al.32 identified cracks and imperfections on the surfaces of the mini-implants that were as received from the manufacturer. Such imperfections were most evident on those mini-implants that, along with the evidence of corrosion, showed consequences of their insertion and removal.
The methodological variability among studies leaves no room for comparison of the results. The present study cannot be compared to others that, for instance, used disks and cylinders, did not control the temperature to reproduce the human body temperature or did not perform the polarisation tests inside a Faraday cage. In order to evaluate the corrosion resistance and surface microstructure of mini-implants used in mechanics that require the anchoring device for a longer period, further studies with mini-implants that have stayed in function for longer than 230 days are required. Our research group is conducting research on mini-implants that were kept in function for a longer period.
Conclusion
The average period of function of 230 days in orthodontic mini-implants made of Ti6Al4V alloy did not cause significant changes in the corrosion resistance and surface characteristics of these devices.�
References
1. McGuire MK, Scheyer ET, Gallerano RL. Temporary anchorage devices for tooth movement: a review and case reports. J Periodontol. 2006;70:1613-24.
2. Park HS, Jeong SH, Kwon OW. Factors affecting the clinical success of screw implants used as orthodontic anchorage. Am J Orthod Dentofacial Orthop. 2006;130:18�25.
3. Chung KR, Choo HR, Kim SH, Ngan P. Timely relocation of mini-implants for uninterrupted full-arch distalization. Am J Orthod Dentofacial Orthop. 2010;138:839-49.
44. Eliades T, Zinelis S, Papadopoulos MA, Eliades G. Characterization of retrieved orthodontic miniscrew implants. Am J Orthod Dentofacial Orthop. 2009;135:10-7.
5. Licausi MP, Munoz AI, Borr�s VA. Influence of the fabrication process and fluoride content on the tribocorrosion behavior of Ti6Al4V biomedical alloy in artificial saliva. J Mech Behav Biomed Mater. 2013;20:137-48.
6. Faverani LP, Bar�o VAR, Pires MFA, Yuan JC, Sukotjo C, Mathew TM, Assun��o WG. Corrosion kinetics and topography analysis of Ti6Al4V alloy subjected to different mouthwash solutions. Mater Sci Eng C Mater Biol Appl. 2014;43:1�10.
7. Lieblich M, Barriuso S, Multigner M, Gonz�lez-Doncel G, Gonz�lez-Carrasco JL. Thermal oxidation of medical Ti6Al4V blasted with ceramic particles: Effects on the microstructure, residual stresses and mechanical properties. J Mech Behav Biomed Mater. 2016;54:173-84.
8. Suzuki MK, Martins DA, Costa MT, Ferreira AC, Ferreira FA. Ions release Evaluation and Changes in Mini-implant Orthodontic Surface. J Contemp Dent Pract. 2018;19:910-7.
9. Prabhu J, Cousley RR. Current products and practice: bone anchorage devices in orthodontics. J Orthod Sci. 2006;33:288�307.
10. Gu�hennec L, Soueida A, Layrolle P. Surface treatments of titani-um dental implants for rapid osseointegration. Dent Mater. 2007;23:844�54.
11. AlSamak S, Bitsanis E, Makou M, Eliades G. Morphological and structural characteristics of orthodontic mini-implants. J Orofac Orthop. 2012;73:58-71.
12. Talha M, Ma Y, Kumar P, Lin Y, Singh A. Role of protein adsorption in the bio corrosion of metallic implants � A review. Colloids Surf B Biointerfaces. 2019;176:494-506.
13. Bagatin CR, Ito IK, Andrucioli MCD, Nelson-Filho P, Ferreira JTL. Corrosion in Haas expanders with and without use of na antimicrobial agent: an in situ study. J Appl Oral Sci. 2011;19:662-7.
14. Abbassy MA, Bakry AS, Zawawi KH, Hassan AH. Long-term durability of orthodontic mini-implants. Odontology. 2018;106:208-14.
15. Do Prado RF, Esteves GC, Santos ELS, Bueno DAG, Cairo CAA, Vasconcellos LGO, Sagnori RS, Tessarin FBP, Oliveira FE, Oliveira LD, Villa�a-Carvalho MFL, Henriques VAR, Carvalho YR, De Vasconcellos LMR. In vitro and in vivo biological performance of porous Ti alloys prepared by powder metallurgy. PLoS One. 2018;13:e0196169.
16. Kao CT, Ding SJ, HE H, Chou MY, Huang TH. Cytotoxicity of orthodontic wire corroded in fluoride solution in vitro. Angle Orthod. 2007;77:349-54.
17. Holst AI, Holst S, Hirschfelder U, Seckendorff VV. Retrieval analysis of different orthodontic brackets: the applicability of electron microprobe techniques for determining material heterogeneities and corrosive potential. J Appl Oral Sci. 2012;20:478-85.
18. Lijima M, Muguruma T, Kawaguchi M, Yasuda Y, Mizoguchi I. In vivo degradation of orthodontic miniscrew implants: surface analysis of as-received and retrieved specimens. J Mater Sci: Mater Med. 2015;26:71-7.
19. Costa MT, Lenza MA, Gosch CS, Costa I, Ribeiro-Dias F. In vitro evaluation of corrosion and cytotoxicity of orthodontic brackets. J Dent Res. 2007;86:441-5.
20. Reis RF, Durante GC. Obten��o de austenita expandida (fase S): Nitreta��o por plasma em baixa temperatura x SHTPN � Parte 2. Rev Mat�ria. 2015;20:316-21.
21. Gil FJ, Delgado L, Espinar E, Llamas JM. Corrosion and corrosion-fatigue behavior of cp-Ti and Ti�6Al�4V lasermarked biomaterials. J Mater Sci Mater Med. 2012;23:885�90.
22. Sampaio M, Buciumeanu M, Henriques B, Silva F, Souza JCM, Gomes JR. Tribocorrosion behavior of veneering biomedical PEEK to Ti6Al4V structures. J Mech Behav Biomed Mater. 2016;54:123-30.
23 Bar�o VA, Mathew MT, Assun��o WG, Yuan JC, Wimmer MA, Sukotjo C. Stability of cp-Ti and Ti-6Al-4V alloy for dental implants as a function of saliva pH � an electrochemical study. Clin Oral Impl Res. 2012;23:1055�62.
24. Knutson KJ, Berzins DW. Corrosion of orthodontic temporary anchorage devices. Eur J Orthod. 2013;35:500-06.
25. Souza JC, Barbosa SL, Ariza EA, Henriques M, Teughels W, Ponthiaux P, Celis JP, Rocha LA. How do titanium and Ti6Al4V corrode in flouridated medium as found in the oral cavity? An in vitro study. Mater Sci Eng. 2015;47:384�93.
26. Azem FA, Delice TK, Ungan G, Cakir A. Investigation of duty cycle effect on corrosion properties of electrodeposited calcium phosphate coatings. Mater Sci Eng C Mater Biol Appl. 2016;68:681-6.
27. Neuman MR. Biopotential electrodes. In: The biomedical engineering handbook. 2th ed. Joseph D, editor. Boca Raton: CRC Press LLC, 2000;47:1-13.
28. Sonntag RE, Gordon JVW. Introdution to thermodynamics: classical and statistical. 3th ed. John Wiley & Sons, 1991;800.
29 Lambert FL. Configurational entropy revisited. J Chem Education. 2007;84:1548-50.
30. Nolan JP. Fluid resuscitation for the trauma patient. Resuscitation. 2001;48:57-69.
31. Risos A, Long N, Hunze A, Gouws G. A 3D Faraday shield for interdigitated dielectrometry sensors and its effect on capacitance. Sensors. 2016;17:77-90.
32. Patil P, Kharbanda OP, Duggal R, Das TK, Kalyanasundaram D. Surface deterioration and elemental composition of retrieved orthodontic . Am J Orthod Dentofacial Orthop. 2015;147:88-100.
�
Marcelo Santos Bahia
Correio eletr�nico: marcelosbahia@outlook.com
�
Acknowledgments
The authors thank FAPEMIG for the support in this study.�
Ethical disclosures
Protection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of data. The authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consent. The authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
�
Conflict of interest
The authors have no conflicts of interest to declare.
�
Article history:
Received 12 February 2019
Accepted 13 May 2019
Available online 29 May 2019
