
Revista Portuguesa de Estomatologia, Medicina Dentária e Cirurgia Maxilofacial
Rev Port Estomatol Med Dent Cir Maxilofac | 2017 | 58 (1) | 8-16
Original research
Ultramorphological and chemical characterization of dentin surfaces after application of two desensitizing toothpastes
Caracterização ultramorfológica e química de superfícies dentinárias após aplicação de duas pastas dentífricas dessensibilizantes
a Área da Medicina Dentária, Faculdade de Medicina da Universidade de Coimbra, Coimbra, Portugal
Alexandra R. Vinagre - avinagre@fmed.uc.pt
Article Info
Rev Port Estomatol Med Dent Cir Maxilofac
Volume - 58
Issue - 1
Original research
Pages - 8-16
Go to Volume
Article History
Received on 05/08/2016
Accepted on 24/11/2016
Available Online on 31/03/2017
Keywords
Original research
Ultramorphological and
chemical characterization of dentin surfaces after application of two
desensitizing toothpastes
Caracterizaçãoultramorfológica e química de superfíciesdentinárias após aplicação de duas pastas dentífricas dessensibilizantes
Alexandra R. Vinagre*, João A. Fagulha, Ana S. Cardoso, Orlando P. Martins, Ana L Messias, João C. Ramos, Isabel P. Baptista
Área da Medicina Dentária, Faculdade de Medicina da Universidade de Coimbra, Coimbra, Portugal
http://doi.org/10.24873/j.rpemd.2017.05.004
Abstract
Objectives:To evaluate dentin tubule obliteration after application of two different desensitizing toothpastes using scanning electron microscopy (SEM) forultramorphologicalanalysis and energy dispersive X-ray spectroscopy (EDX) for chemical evaluation.
Methods: Five dentin discs were sectioned into four quarters; surfaces were etched with 6% citric acid for 2 minutes and equally distributed into four groups. In G1 (control) no treatment was performed; dentin surfaces were brushed twice-daily during 14 days with artificial saliva (G2), a combined stabilized stannous fluoride, sodiumhexametaphosphateand silica (SFSH) toothpaste (G3) and a calcium sodiumphosphosilicate(CSFS) toothpaste (G4), under a standardized protocol. All specimens were analyzed by SEM and EDX and tubule occlusion was scored. Statistical analysis was performed usingKruskal-Wallis (p<0.05).
Results: Score distribution across groups consistently increased from G1 to G4, being the last the most consistent group. Statistical between-treatment comparisons for 750-fold and 2000-fold magnification revealed significant differences between groups (p=0.009 and p=0.002, respectively). For both magnifications, post-hoc analysis adjusted for multiple comparisons only indicated statistically significant differences between G1 and G4 (p=0.012 and p=0.001, respectively). Chemical analysis revealed high levels of carbon, oxygen and nitrogen for G1. For G2 an increase of the levels of the phosphorous and calcium elements and a drop of oxygen and carbon levels was registered. G3 and G4 showed a surface layer mainly composed of calcium and phosphorous.
Conclusions:Both desensitizing toothpastes induced high levels of tubule occlusion with consistent phosphorus and calcium deposition over dentin surface.
Keywords:Dentin hypersensitivity, Dentin tubules, Energy dispersive X-ray, Scanning electron microscopy, Toothpaste.
Resumo
Objectivos:Avaliar aobliteracaodostubulosdentinariosaposaaplicacaode duas pastasdentifricasdessensibilizantes, usando microscopiaeletronicade varrimento (MEV) e espectroscopia de raios X pordispersaoem energia (EDX).
Métodos:Foram seccionados cinco discos de dentina em quatro quartos, condicionados comacidocitricoa 6% por 2 minutos edistribuidosequitativamente em quatro grupos: No G1 (controlo)naoseefetuouqualquer tratamento; assuperficiesdentinariasforam escovadas duas vezes por dia durante 14 dias com saliva artificial (G2), uma pasta de fluoretoestanhosoestabilizado, hexametafosfato desodioesilica(SFSH) (G3) e uma pasta defosfosilicatodesodioecalcio(CSFS) (G4). As amostras de cada grupo foram quantificadas por MEV e EDX. Na analiseestatisticaaplicou-se o teste deKruskal-Wallis(p<0,05).
Resultados:O grau deoclusaotubular aumentou de G1ateG4, sendo este ultimo o maisconsistente.Paraasampliacoesde 750x e 2000x foram encontradasdiferencasestatisticamente significativas entre grupos (p=0,009 e p=0,002,respetivamente). Em ambos os casos a analisepost-hoc ajustada paracomparacoesmultiplasapenas identificoudiferencasentre G1 e G4 (p=0,012 e p=0,001,respetivamente). Aanalisequimicarevelou elevados níveis de carbono,oxigenioenitrogeniono G1. Para o G2 registou-se um aumento dosniveisdefosforoecalciocom umadiminuicaoconcomitante deoxigenioe carbono. Os G3 e G4 apresentavam maioritariamentefosforoecalcio.
Conclusões:Ambas as pastas dessensibilizantes induziram um elevado grau deoclusaotubular com umadeposicaoconsistente defosforoecalciosobre asuperficiedentinaria.
Palavras-chave:Hipersensibilidade dentária,Tubulosdentários,Raios X pordispersaoem energia,Microscopia electrónica,de varrimento,Pasta dentífrica,
Introduction
Dentin hypersensitivity (DH) is defined as a short, sharp pain arising from exposed dentin in response to typically thermal, evaporative, tactile, osmotic or chemical stimuli.1 DH-related discomfort may have a significant negative impact on an individual’s daily life, as it may cause difficulties in eating, drinking and speaking.2 Due to its high prevalence, significant efforts have been made to understand the etiology and mechanisms involved in DH development.3 Several conditions were identified, among them: gingival recession; periodontal disease; deep tooth cracks and loss of enamel,cementum, and dentin due to mechanical abrasion, chemical erosion, and tooth fracture.4 ,5
A common feature of DH is the presence of open dentin tubules, which provide a direct link between the external environment and the tooth pulp.6Thereare a large number of options for managing DH using chemical or physical agents.
Current treatments tend to concentrate on two approaches: neural transmission blockage or tubule occlusion.7 Recently, two new promising molecules were developed for hypersensitivity management: stabilized stannous fluoride containing sodiumhexametaphosphate(SFSH) and a calcium sodiumphosphosilicate(CSPS).
Stannous fluoride has been incorporated in dental dentifrices due to its therapeutic behavior in different fields, such as protection against carious pathogenic bacteria, gingivitis, hypersensitivity and plaque development.8However,its clinical usage was limited because of astringent taste and extrinsic staining of the teeth. Those limitations were outdated when a novel dentifrice introduced a new formulation combining stabilized stannous fluoride, sodiumhexametaphosphate, and silica (SFSH). This formula offers the therapeutic benefits of a 0.454% stabilized stannous fluoride and stain-control characteristics of sodiumhexametaphosphatein a low-water formulation dentifrice.9When this anhydrous preparation is applied on dentin surfaces the occlusion of tubules by a tin-rich low solubility complexes is expected.10
Calcium sodiumphosphosilicate(CSPS) is an inorganic amorphous compound that contains calcium, sodium, phosphate and silica.11When CSPS particles contact an aqueous environment, an immediate release of sodium ions occurs, which increases local pH environment. The surface reaction include the ion exchange between Na2+ from CSPS and H+ from dentin fluid resulting in the formation of a porous silica rich layeron the surface, that provides a nucleating site for early precipitation of a calcium phosphatehydroxycarbonateapatite layer.12
The aim of thisin vitrostudy was to evaluate the effectiveness of two desensitizing dentifrices, SFSH- and CSPS-based, in occluding dentinal tubules using scanning electron microscopy (SEM). The null hypothesis is that there are no differences regarding dentin tubule occlusion between the materials tested.
Material and methods
Five caries free human third molars were collected after obtaining patient informed consents, as approved by the Ethical Committee. The teeth were cleaned and stored at room temperature in a 10% buffered formalin solution (pH 7.0). Five dentin discs of 1 mm thickness were obtained by sectioning each tooth parallel to theocclusalsurface from the top of the pulp horns andocclusallyusing a hard tissue cutting saw (Accutom50,Struers,Ballerup, Denmark), with water as coolant.
Each disc was then sectioned into four quarters that have been identified and singly stored in artificial saliva until required (Figure 1).
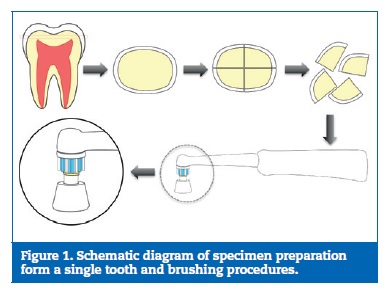
All specimens had their smear layer removed byultrasonicationin deionized water followed by surface etching with 6% citric acid for 2 minutes and rinsing with distilled water for 30 seconds to create opened dentin tubules. The specimens from eachtooth were equally distributed into four groups, each containing five samples (n=5). In Group 1 (control), samples were immersed in artificial saliva for 14 days. For the other groups, a single-tuft toothbrush mounted in an electric brushing device (Oral-BR ProfessionalCareR500, Procter & Gamble Co., Cincinnati, OH, USA) was applied perpendicular to the dentin surface (Figure 1), at a constant loading for 30 seconds, twice daily (12 hours interval) for 14 days. In G2, samples were brushed with 4 ml of artificial saliva, whilst in G3 and G4 specimens were brushed with 40 g of the respective toothpaste, SFSH (Oral-BR Pro Expert; Procter & Gamble UK,Weybridge, UK) and CSPS (SensodyneR Repair&Protect; GlaxoSmithKline; Slough, UK), without any dilution. After brushing, samples were gently rinsed with 10 ml of deionized water for 10 seconds, and stored in artificial saliva at 37.oC, until used in the next brushing session. Artificial saliva was changed between all brushing periods. (Table 1)
For SEM analysis, the samples were fixed in 2.5%glutaraldehydein 0.1 M phosphate buffer for 48h at room temperature.
The specimens were then dehydrated in ascendant alcohol solutions (50%, 75% 90%, 95%, 100%) and submitted to chemical drying inhexamethyldisilazane. All samples were mounted on aluminum stubs using carbon sticky pads, sputter coated withgold and subjected to SEM analysis (JSM 5310, JEOL; Tokyo, Japan). The acceleration voltage was set at 10 kV. To achieve a cross section view, samples were then fractured into halves. To assess the level of tubule occlusion, photomicrographs were taken from each dentin surface at a 750-fold and 2000-fold magnification. Image grading was performed based on those micrographs, by two blinded evaluators once and independently.
When disagreements arose, the examiners had to reach a consensus. Evaluation was undertaken according to a six-point scale:
– Score 1 (Sc1): open tubules;
– Score 2 (Sc2): most tubules open (~90%);
– Score 3 (Sc3): half of tubules occluded (~50%);
– Score 4 (Sc4): most tubules occluded but tubules outlines visible;
– Score 5 (Sc5): most tubules occluded (~90%);
– Score 6 (Sc6): all tubules occluded.
Additionally, energy dispersive X-ray (EDX) analysis was performed from two surface samples of each group. The acceleration voltage of the scanning electron microscope was set to 20 kV and EDX spectra were collected using a Si-detector (X-MaxNdetector, Oxford Instruments,Oxfordshire, UK). Spectra were processed usingAZtecEnergyanalysis software (AZtec, Oxford Instruments,Oxfordshire, UK) for surface element composition detection and for relative element contents calculation in weight percentage.
Statistical analysis was performed using IBM®SPSS Statistics Version 20.0 (SPSS, Chicago, IL, USA). Non-parametric group comparison was performed usingKruskal-Wallis and all pairwise as post-hoc comparisons. Wilcoxon signed-ranks test was applied for intragroup comparison at 750-fold and 2000-fold magnifications. Significance level was set at 0.05.
Results
The between-observers agreement was quantified byintraclasscorrelation coefficient analysis for single measures that showed a high level of agreement (ICC= 0.937,p<0.01).
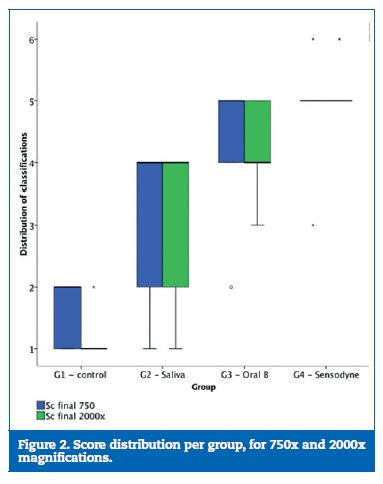
Statistical between-treatment comparisons of the occlusion scores mean ranks for 750-fold and 2000-fold magnification revealed significant differences between groups (p=0.009 and p=0.002, respectively). For 750-fold and 2000-fold magnification, post-hoc analysis adjusted for multiple comparisons only indicated statistically significant differences between G1 and G4 (p=0.012 and p=0.001, respectively). However, for both magnifications, G1 showed the lowest mean score indicating the least percentage of tubule occlusion while G4 showed the maximum mean score, with the highest level of occlusion and the most consistent results in the final score distribution (Figure 2) Wilcoxon signed ranks test showed no differences between scores obtained at the 750-fold and the 2000-fold magnification (Z= -0.087,p=0.931).
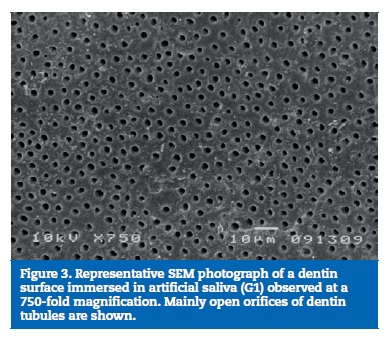
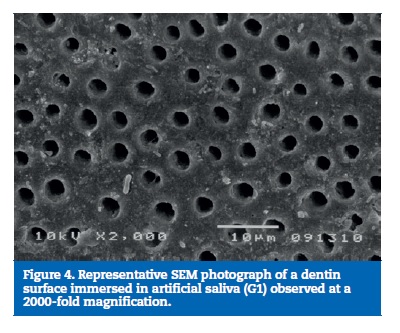
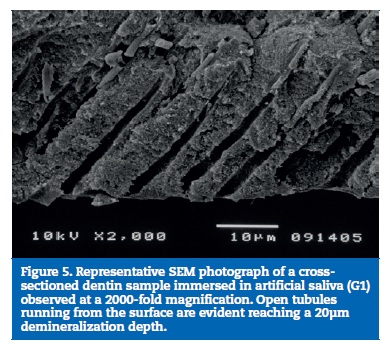
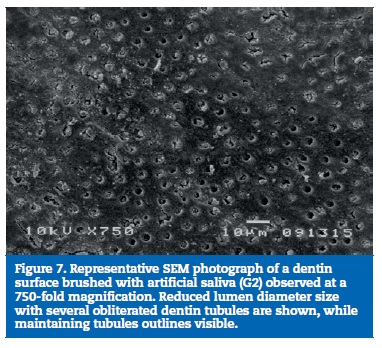
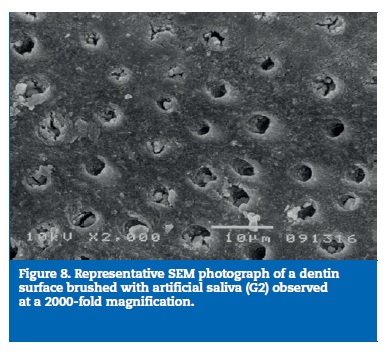
All samples of G1 showed open dentin tubules (Figures 3-6). In G2, samples showed a reduction in the tubule lumen diameter and a considerable number of obliterated tubules, while maintaining tubules outlining remained mostly visible. Tubule entrance was occasionally filled with precipitates with no more than a 2μmdepth (Figures 7-10). G3 dentin surfaces presents an incomplete surface coating layer where a great number of dentinal tubules became partially or completely obliterated and precipitates occlude tubule lumens in an inhomogeneous form (Figures 11-14). In G4, most surfaces appeared completely covered by an irregular layer with few or no open tubules discernible occluded with precipitates (Figures 15-18).


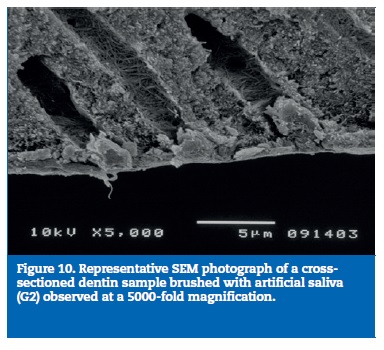








According to the EDX analysis, relative element contents calculation in weight percentage (wt.%) obtained for each group are shown in Table 2. G1 showed high levels of carbon, oxygen, and nitrogen. For G2, chemical mapping showed an increase of the levels of the phosphorous and calcium elements and a simultaneous dropof carbon levels. For G3, EDX showed that surface layer was mainly composed of calcium and phosphorous.
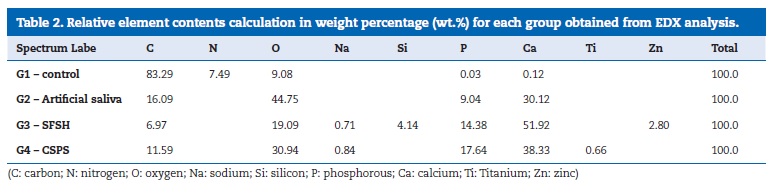
Additionally, signs of silicon, zinc and sodium were also found. For G4 EDX showed that occlusion deposits were predominantly composed of calcium and phosphorous. Additionally, discrete signs of titanium and sodium were also found.
Discussion
Dentin hypersensitivity is considered one of the most prevalent painfulconditionof the oral cavity, but it is poorly understood. The ideal treatment has not yet been reached and no goldstandard treatment has been advocated.13 ,14
The dentin disc model has been used in several earlier studies and was considered to represent a close approximation of thein vivosituation.15 In order to improve this model, homogenization of dentin substrate across groups was ensured, as dentin substrates can exhibit different features from tooth to tooth.16 -18
SEM analyses were performed using 750-fold and 2000-fold magnifications as reported in other studies.19 -22 Nevertheless, the use of a 750-fold magnification can be considered more reliable for precise evaluation, as a relatively broad image is available for accurate quantification.
In some instances, natural occlusion of tubules occur due to smear layer formation or calcium phosphate deposits mainly derived from saliva.23 However, these occlusions may be easily modified by tooth brushing or acid challenge, dislodging the tubule obstruction. This may explain why the DH condition is related to frequent episodes of acute pain followed by periods of quiescence.23 Infact,G2 showed a considerable number of obliterated tubules, which can be attributed to calcium phosphate precipitation on the dentin surface, because artificial saliva is supersaturated with respect to hydroxyapatite.24
Both toothpastes induced a well-developed occluding ability. Therefore, the null hypothesis could not be rejected. Nevertheless, the occlusion pattern tended to be higher and more homogeneous for the CSPS.
Quantitative SEM score results and chemical analyses for CSPS toothpaste obtained in the present study was similar to those reported by others authors. 25 -28 The mineralized surface layer detected in treated samples was previously described as a mixture ofnano-crystalline and amorphous material, composed by a hydroxyapatite-like residue, resulting in the formation of a calcium-phosphate enriched layer resistant to acid and mechanical challenges.25 ,26 ,29 This stabilized surface layer can result in tubular occlusion, but also in the potential chemical interaction of CSPS with exposed type I collagen fibers.30
Accordingly, the EDX surface map evidenced the main presence ofCaand P, as expected. Low levels of Ti and Na were also detected and the Ti signal is thought to result from TiO2 formation.25Theseresults are consistent with clinical findings reported in the literature indicating that CSPS is an effective agent for reducing tooth sensitivity assessed by randomized controlled clinical trials and in a recent meta-analysis.31 -36
Concerning the SFSH dentifrice, few studies were published.
An experimental study showed that when a stannous fluoride anhydrous preparation was brushed in dentin, a nearly complete coverage of the dentine surface and occlusion of tubules by a tin-rich surface deposit was observed.10In the present study, high contents ofCaand P were found along with small levels of Si, Zn and Na. The Si and Zn signals should have resulted from SiO2 and ZnO2 formation.24 Surprisingly, the tin element was not identified in spectra, although a discrete peak at the typical tin-specific Lαline at 3.443keVhas been detected.37Similar results were obtained byGansset al.38, whose work showed that the amount of tin retained in sound dentin and on surfaces where the organic matrix was preserved was much lower than on dentin surfaces that underwent severe erosive conditions. Besides, they reported a considerably thick continuous layer covering sound dentin surface, consisting mainly onCaand P and relatively small amounts of tin, emphasizing that the mechanism behind this covering is unclear. No accurate conclusions can be drawn on the source of theCa, P and O signals, but it may be related to a slight demineralization of organic matrix.40 It is possible that the tin signal could be found more deeply in dentin, as tin uptake is related to a dose-dependent diffusion control deep through the collagen structure. The chemical interaction with tin can occur either with the mineral content by the formation of tin salts and/or with collagen or other dentin protein that contains negatively charged groups, capable of bindingcationswith high affinity.38 Therefore, the occlusion of tubule lumens that became evident after brushing treatment with SFSH toothpaste, could be due to both pathways.
Severalin vivoreports showed that dentifrices or gels containing stannous fluorides had a significant effect in reducing sensitivity in the long-term.39 -42
In order to improve the experimental methodology, further studies should be performed with an increase in sample size using lower magnifications for evaluation, while subjecting samples to daily erosive or mechanical challenges. These features would allow a more accurate simulation of real clinical conditions.
Conclusions
According to the presentin vitrostudy no statistically significant differences on the occlusion ability of dentin tubules were found between SFSH and CSPS toothpastes. However, a more homogenous dentin occlusion was achieved with CSPS dentifrice.
Brushing with artificial saliva produced a more limited dentin tubule occlusion when compared to the toothpastes.
From EDX analysis, brushing either with SFSH or CSPS conduces to high levels of phosphorus and calcium deposition over dentin tubules.
References
1. West NX,LussiA,SeongJ,HellwigE. Dentin hypersensitivity: pain mechanisms andaetiologyof exposed cervical dentin.ClinOralInvestig.2013;17(Suppl1):9-19.
2.AjcharanukulO,KraivaphanP,WanachantararakS,VongsavanN, Matthews B. Effects of potassium ions on dentine sensitivity in man. Arch Oral Biol. 2007;52:632-9.
3. Li Y. Innovations for combating dentin hypersensitivity: current state of the art.CompendContinEducDent.2012; 33(Spec No 2):10-6.
4.DavariAR,AtaeiE,AssarzadehbH. Dentin Hypersensitivity: Etiology, Diagnosis and Treatment; A Literature Review. J Dent(Shiraz). 2013;14:136-45.
5. Canadian Advisory Board on Dentin Hypersensitivity.Consensus-based recommendations for the diagnosis and management of dentin hypersensitivity.J Can Dent Assoc. 2003;69:221-6.
6.BartoldPM. Dentinal hypersensitivity: a review.AustDent J. 2006;51:212-8;quiz 276.
7.MiglaniS,AggarwalV,AhujaB. Dentin hypersensitivity: Recent trends in management.JConservDent.2010; 13:218-24.
8.BaigA, He T. A novel dentifrice technology for advanced oral health protection: A review of technical and clinical data.CompendContinEducDent.2005;26(9Suppl1):4-11.
9.SensabaughC,SagelME. Stannous fluoride dentifrice with sodiumhexametaphosphate: review of laboratory, clinical and practice-based data.J DentHyg.2009;83:70-8.
10. Miller S, Truong T,HeuR,StranickM, Bouchard D,GaffarA. Recent advances in stannous fluoride technology: antibacterial efficacy and mechanism of action towards hypersensitivity.IntDent J. 1994;44(1Suppl1):83-98.
11. Layer TM.Development of aFlouridated, Daily-Use Toothpaste ContainingNovaMinTechnology for the Treatment of Dentin Hypersensitivity.JClinDent.2011; 22:59-61.
12.AnderssonOH,KangasniemiI. Calcium phosphate formation at the surface of bioactive glass in vitro. J Biomed Mater Res. 1991;25:1019-30.
13.BanoczyJ. Dentine hypersensitivity: general considerations for successful practice management.IntDent J. 2002;52:366.
14.VinayaKR,ShubhashiniN,SeshanH,KrantiK. A clinical trial comparing a stannous fluoride based dentifrice and a strontium chloride based dentifrice in alleviating dentinal hypersensitivity.JIntOral Health.2010;2:37-50.
15.PurraAR,MushtaqM. Scanning electron microscopic evaluation of the desensitizing effect ofpropolisin the dentine disc model: An in-vitro study.IntJ Dent Oral Health.2012;4:6-9.
16.MordanNJ, Barber PM,GillamDG.The dentine disc.A review of its applicability as a model for the in vitro testing of dentine hypersensitivity.J OralRehabil.1997;24:148-56.
17. Marshall GW Jr. Dentin: microstructure and characterization. Quintessence Int. 1993;24:606-17.
18.MjorIA,NordahlI.The density and branching of dentinal tubules in human teeth.Arch Oral Biol. 1996;41:401-12.
19.OlleyRC,PileckiP, Hughes N, Jeffery P, Austin RS,MoazzezR, Bartlett D.An in situ investigating dentin tubule occlusion of dentifrices following acid challenge.J Dent. 2012;40: 585-93.
20.GillamDG, Tang JY,MordanNJ, Newman HN. The effects of a novelBioglassdentifrice on dentine sensitivity: a scanning electron microscopy investigation.J OralRehabil.2002;29:305-13.
21.PetrouI,HeuR,StranickM,etal.A breakthrough therapy for dentin hypersensitivity: how dental products containing 8% arginine and calcium carbonate work to deliver effective relief of sensitive teeth.JClinDent.2009;20:23-31.
22. West NX,AddyM,NewcombeR, et al. A randomized crossover trial to compare the potential of stannous fluoride and essential oil mouth rinses to induce tooth and tongue staining.ClinOralInvestig.2012;16:821-6.
23.AddyM. Dentin hypersensitivity: newprespectiveson an old problem.IntDent J. 2007;57:367-75.
24.ArraisCA,MicheloniCD,GianniniM, Chan DC.Occluding effect of dentifrices on dentinal tubules.J Dent. 2003;31:577-84.
25. Earl JS, Leary RK, Muller KH, Langford RM, Greenspan DC.Physical and chemicalcharaterizationof dentin surface following treatment withNovaMintechonology.JClinDent.2011;22:62-7.
26. Parkinson CR,WillsonRJ.Acompartivein vitro study investigating the occlusion and mineralization properties of commercial toothpaste in a four-day dentin disc model.JClinDent.2011;22:74-81.
27. Burwell A, Jennings D, Muscle D, Greenspan DC.NovaMinand dentin hypersensitivity–in vitro evidence of efficacy.JClinDent.2010;21:66-71.
28.GjorgievskaES, Nicholson JW.A preliminary study of enamelremineralizationby dentifrices based onRecalden(CPP-ACP) andNovamin(calcium-sodium-phosphosilicate).ActaOdontolLatinoam.2010;23:234-9.
29. West NX, Macdonald EL, Jones SB,ClaydonNC, Hughes N, Jeffery P. Randomized in situ clinical study comparing the ability of two new desensitizing toothpastetechonologiesto occlude patent dentin tubules.JClinDent.2011;22:82-9.
30.EfflandtSE,MagneP, Douglas WH, Francis LF.Interaction between bioactive glasses and human dentin.J MaterSciMater Med. 2002;13:557-65.
31.NarongdejT,SakoolnamarkaR,BoonroungT.The effectiveness of a calcium sodiumphosphosilicatedesensitizer in reducing cervicaldentinhypersensitivity.J Am Dent Assoc. 2010;141:995-9.
32.PradeepAR, Sharma A. Comparison of clinical efficacy of adentrifricecontaining calcium sodiumphosphosilicateto a toothpaste containing potassium nitrate and a placebo on dentinal hypersensitivity: a randomized clinical trial.JPeriodontol.2010;81:1167-73.
33.LitkowskiL, Greenspan DC.A clinical study of the effect of calcium sodiumphosphosilicateon dentin hypersensitivity–proof of principle.JClinDent.2010;21:77-81.
34. Du Min Q,BianZ, Jiang H, Greenspan DC, Burwell AK,ZhongJ, TaiBJl. Clinical evaluation of a dentifrice containing calcium sodiumphosphosilicate(novamin) for the treatment of dentin hypersensitivity. Am J Dent. 2008;21:210-4.
35. Salina S, Thakur S,KulkarniS, La Torre G. A randomized, controlled clinical study evaluation theeffecacyof two desensitizing dentifrices.JClinDent.2010;21:82-7.
36. Zhu M, Li J, Chen B, Mei L, Yao L,TianJ, Li H, The effect of Calcium SodiumPhosphosilicateon Dentin Hypersensitivity: A Systematic Review and Meta-Analysis.PLoSOne.2015;10:e0140176.
37.SchlueterN,HardtM,LussiA, Engelmann F,KlimekJ,GanssC. Tin-containing fluoride solutions as anti-erosive agents in enamel: an in vitro tin-uptake, tissue-loss, and scanning electron micrograph study. J Oral Sci. 2009;117:427-34.
38.GanssC,HardtM,LussiA, Cocks AK,KlimekJ,SchlueterN. Mechanism of action of tin-containing fluoride solutions as anti-erosive agents in dentine – an in vitro tin-uptake, tissue loss, and scanning electron microscopy study. J Oral Sci. 2010;118:376-84.
39. Sharma N, Roy S,KakarA, Greenspan DC, Scott R.A clinical study comparing oral formulations containing 7.5% calcium sodiumphosphosilicate(NovaMin), 5% potassium nitrate, and 0.4% stannous fluoride for the management of dentin hypersensitivity.JClinDent.2010;21:88-92.
40. Schiff T,SalettaL, Baker RA, Winston JL, He T. Desensitizing effect of a stabilized stannous fluoride/Sodiumhexametaphosphatedentifrice.CompendContinEducDent.2005;26(9Suppl1):35-40.
41. Thrash WJ,DoddsMW, Jones DL.The effect of stannous fluoride on dentinal hypersensitivity.IntDent J. 1994; 44(1Suppl1):107-18.
42. Thrash WJ JD,Dodds WJ. Effect of a fluoride solution on dentinal hypersensitivity.Am J Dent 1992;5:299-302.
Alexandre Vinagre
E-mailaddress: avinagre@fmed.uc.pt
Ethical disclosures
Protection of human and animal subjects.The authors declarethat no experiments were performed on humans or animalsfor this study.
Confidentiality of data.The authors declare that no patient data appear in this article.
Right to privacy and informed consent.The authors declare that no patient data appear in this article.
Conflict of interest
The authors have no conflicts of interest to declare.
Acknowledgements
The authors would like to express their gratitude to Dr FranciscoBastoandEngAntonio Fonseca for their technical support.
Article History:
Received 5 August 2016
Accepted 24 November 2016
Available online 30March2017
