
Revista Portuguesa de Estomatologia, Medicina Dentária e Cirurgia Maxilofacial
SPEMD | 2017 | 58 (4) | 205-211
Original research
The use of systemic antibiotics in endodontics: a cross-sectional study
O uso sistémico de antibióticos em endodontia: estudo transversal
a Universidade Católica Portuguesa - Polo Regional das Beiras – Viseu, Portugal
b CIIS – Centro de Investigação Interdisciplinar em Saúde – Universidade Católica Portuguesa
Article Info
Rev Port Estomatol Med Dent Cir Maxilofac
Volume - 58
Issue - 4
Original research
Pages - 205-211
Go to Volume
Article History
Received on 29/04/2016
Accepted on 20/11/2017
Available Online on 17/01/2018
Keywords
Original research
The use of systemic antibiotics in endodontics: a cross-sectional study
O uso sistémico de antibióticos em endodontia: estudo transversal
Miguel Silvaa, Manuel Pauloa, Miguel Cardosoa, Miguel Martinsa, Rita Noitesa,b,*
a Universidade Católica Portuguesa - Polo Regional das Beiras – Viseu, Portugal
b CIIS – Centro de Investigação Interdisciplinar em Saúde – Universidade Católica Portuguesa
http://doi.org/10.24873/j.rpemd.2017.12.033
ABSTRACT
Objectives: Portugal is one of the European countries with the highest antibiotic consumption rate and, consequently, the highest rates of bacterial resistance. Dentistry’s contribution to that problem can be substantial because dentists prescribe approximately 10% of all common antibiotics. The purpose of this study was to characterize the prescription of systemic antibiotics for pulpal and periapical pathology in a sample of Portuguese dentists.
Methods: A cross-sectional study was conducted in dentists working in the city of Viseu. A total of 135 questionnaires were distributed among all dental clinics and dental offices of Viseu.
Results: The overall response rate was 70% (n = 95). The vast majority of dentists prescribed antibiotics for 8 days (78.9%). The most commonly prescribed antibiotic therapy was the association 875-mg amoxicillin with 125-mg clavulanic acid (82.1%). In cases of sensitivity to penicillin, the most prescribed antibiotics were 500-mg clarithromycin (34.7%) and 500-mg azithromycin (33.7%). A considerable percentage of dentists prescribed antibiotics for situations of irreversible pulpitis, pulp necrosis without systemic involvement, fistula and endodontic retreatment.
Conclusions: A considerable part of the inquired dentists prescribed antibiotics inappropriately for endodontic inflammatory conditions such as pulpitis. This kind of behavior could contribute to the world problem of antimicrobial resistance. It is important that dentists understand the importance of restricting the use of antibiotics for cases of severe infection, when they are truly needed.
Keywords: Antibiotic therapy, Antimicrobial resistance, Endodontic infections, Prescription habits, Systemic antibiotics
RESUMO
Objetivos: Portugal é um dos países europeus com maior taxa de consumo de antibióticos e, consequentemente, com as maiores taxas de resistência bacteriana. Os médicos dentistas podem contribuir de forma substancial para esse problema, sendo da sua responsabilidade a prescrição de aproximadamente 10% de todos os antibióticos comuns. O objetivo deste estudo foi caracterizar os hábitos de prescrição de antibióticos sistémicos para a patologia pulpar e periapical numa amostra de médicos dentistas portugueses.
Métodos: Foi realizado um estudo transversal envolvendo os médicos dentistas da zona geográfica de Viseu. Um total de 135 questionários foi distribuído por todos os consultórios e clínicas médico-dentárias de Viseu.
Resultados: A taxa de resposta foi de 70% (n=95). A grande maioria dos médicos dentistas prescreve antibióticos por 8 dias (78,9%). O antibiótico mais frequentemente prescrito foi a associação de amoxicilina com ácido clavulânico 875 / 125 mg (82,1%). Em caso de sensibilização à penicilina, os antibióticos mais prescritos foram a claritromicina 500 mg (34,7%) e azitromicina 500 mg (33,7%). Verificaram-se percentagens consideráveis de abuso de antibióticos em situações de pulpite irreversível, necrose pulpar sem envolvimento sistémico, fístula e em casos de retratamento endodôntico.
Conclusões: Uma parte considerável dos médicos dentistas inquiridos prescreve antibióticos inadequadamente para condições endodônticas inflamatórias como a pulpite. Este tipo de comportamento pode contribuir para o problema mundial da resistência antimicrobiana. É importante que o Médico Dentista compreenda a importância de restringir o uso de antibióticos aos casos de infeção grave que necessitam deles.
Palavras-chave: Terapêutica antibiótica, Resistência antimicrobiana, Infeções endodônticas, Hábitos de prescrição, Antibióticos sistémicos
Introduction
Despite having a national Program for Prevention and Control of Infections and Antimicrobial Resistance, Portugal is one of the European countries with the highest rates of antibiotic consumption and, consequently, highest rates of bacterial resistance. 1 - 3 One of the main reasons for the increase in bacterial resistance is the overuse of these drugs by health professionals, which means that there is currently a major concern about the therapeutic abuse of antibiotics. 4 - 7 Dentists can contribute to the problem of antimicrobial resistance substantially because they prescribe approximately 10% of all common antibiotics. 4
Bacterial resistance is defined as the ability of a microorganism to withstand the effects of antibiotics in the presence of concentrations higher than those of therapeutic doses in humans. 8 - 11 Currently, microorganisms are recognized as the etiological agents of virtually every pulp and periapical disease, 11 and it is consensual that endodontic infections are polymicrobial and involve a combination of gram‑positive, gram‑negative, facultative anaerobic and strictly anaerobic bacteria. 12 13
As professionals qualified to prescribe these drugs, dentists should evaluate the real need for using antibiotics. It is estimated that, in around 60% of cases of infections in humans, the host’s own defenses are responsible for solving the process without the need for antibiotics. 4 , 14 The purpose of the present study was to characterize the systemic antibiotic prescription habits for pulp and periapical pathologies in a sample of Portuguese dentists. Based on the results observed in previous studies, 9 , 10 , 12 we hypothesize to find a higher percentage of antibiotic use in our sample.
Materials and Methods
This observational, analytical, cross‑sectional survey was carried out between January and June 2016. The target sample comprised the dentists that worked in clinical practice in the city of Viseu. This study follows the STROBE guidelines for reporting the results of observational studies.
A questionnaire about personal and professional characteristics, as well as specific questions regarding the prescription of antibiotics, was developed and sent to all dentists of the city of Viseu that were registered in the database of the Portuguese Dental Association (Ordem dos Médicos Dentistas).
The questionnaire was developed based on previous surveys on this topic conducted in the USA,12 Spain 9 , 10 and Brazil. 15
The questionnaire was sent to three dentists experts on the field, who were asked to comment on the layout and content.
Some amends were made in light of their comments. The questionnaire began with the description of the objective of the study and the ethical considerations concerning anonymity and confidentiality of data. The questions were divided into two main groups. The first group referred to the sample characterization with questions regarding personal (age and gender) and professional characteristics, intended to obtain information about the dentists’ education degree, years of clinical practice and number of weekly endodontic treatments. The second group of questions concerned the prescription of antibiotics in their clinical practice, namely, the type of antibiotics (for patients with and without allergies), the clinical conditions in which they were used and the number of days of treatment. All the participants were contacted in person. All data were analyzed using the IBM SPSS Statistics 20 software (IBM Corporation, Chicago, IL, USA). Sample characteristics were analyzed as average ± standard deviation (SD), counts or proportions. The χ2 test was used for comparisons and correlations between groups in nominal data. The Spearman’s rank correlation coefficient was also used to test for correlations. The differences in the prescribed number of days for antibiotic treatment between males and females and between different academic degrees (DDS degree, master or PhD) were tested with independent t‑tests. The differences in the prescribed number of days for antibiotic treatment between age groups and between groups of dentists with different experience/time dedicated to endodontics practice was tested with the one‑way ANOVA. The level of significance was set at p < 0.05.
Results
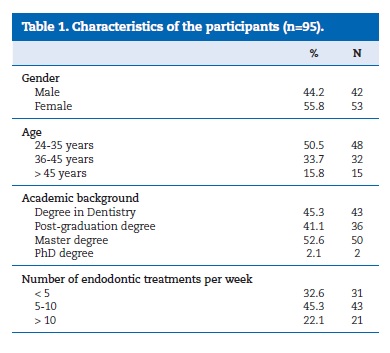
From a total of 135 questionnaires, 95 dentists (females: 55.8%) participated in this study. Table 1 summarizes the characteristics of the participants. Fifty percent of the dentists were less than 35 years old. The participants had an average of 10 years (SD = 7.5 years) of clinical experience. Most of the dentists held a master degree and more than 40% held a post‑graduate degree (Table 1). The most common number of endodontic treatments performed per week was 5 to 10 (Table 1).
Regarding antibiotic prescription, the great majority of dentists referred prescribing antibiotic therapy for 8 days (78.9%); 13.7% for 7 days, 3.2% for 10 days, 3.2% for 5 days and 1.1% for 6 days.
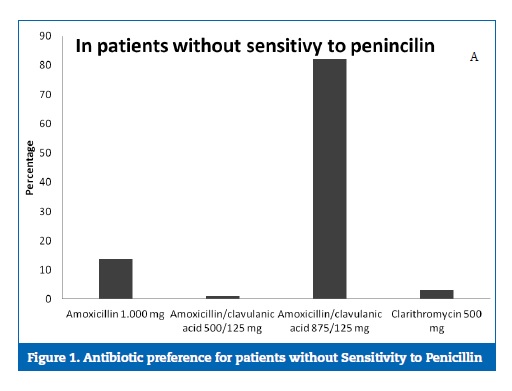
Most of the dentists (82.1%) prescribed 875‑mg amoxicillin associated with 125‑mg clavulanic acid in patients with no medical allergies (Figure 1). The second most often prescribed antibiotic was amoxicillin (13.7%).
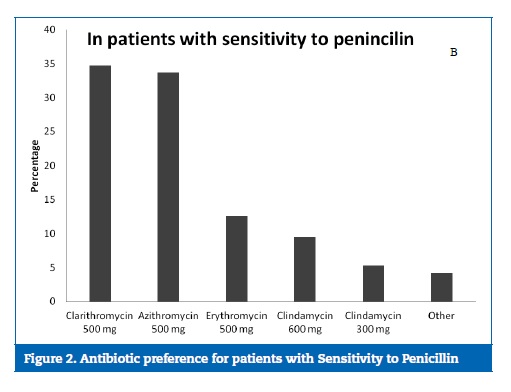
Clarithromycin and azithromycin were the first‑choice antibiotics for patients with sensitivity to penicillin, as they were prescribed by 34.7% and 33.7% of the dentists, respectively (Figure 2).
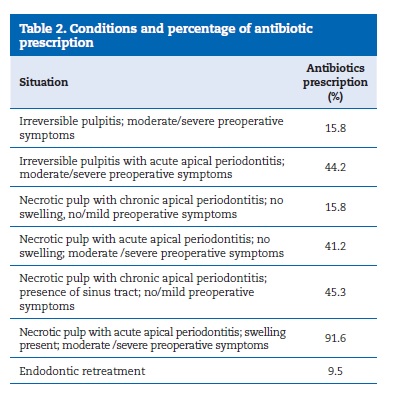
We observed that most professionals prescribed antibiotics in cases of necrotic pulp, acute apical periodontitis, swelling, and other moderate/severe symptoms (91.6%).
In cases of necrotic pulp and chronic apical periodontitis where the patient was asymptomatic but had a sinus tract, 45.3% of the dentists prescribed antibiotics (Table 2). In cases of necrotic pulp and chronic apical periodontitis with no swelling and no other symptoms, antibiotics were prescribed by 15.8% of the dentists. The percentages of antibiotic prescription for other pulpal and periapical conditions are described in Table 2.
There were no significant differences in the prescribed number of days for antibiotic treatment in relation to age, sex, academic degree, or experience/time dedicated to endodontics practice as expressed by the number of treatments performed per week (Table 3).
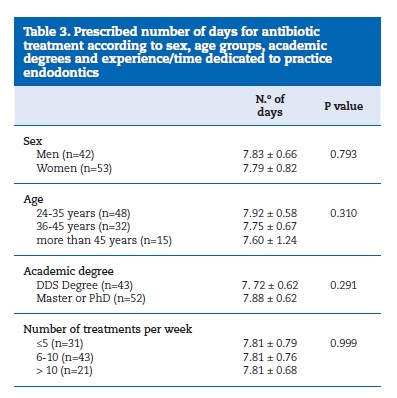
No correlations were found between antibiotic prescription and sex (p=0.570), clinical experience (p=0.399), number of endodontic treatments performed per week (p=0.199) or having a post‑graduation (p=0.147), a master (p=0.611) or a PhD degree (p=0.931). A tendency for younger dentists to prescribe more antibiotics was observed (p=0.079), although without reaching statistical significance.
Discussion
Endodontic infections typically have a rapid onset and a short duration of up to 2 to 7 days, particularly if the cause is treated or eliminated. 16 , 17 In our study, the average length of antibiotic prescriptions was 7.81 days, with a range of 5 to 10 days. Comparing to the average treatment duration reported in other similar studies, 9 , 10 , 12 , 15 the respondents in this study prescribed for longer periods.
The proper dose and duration of an antibiotic treatment are enough when there is sufficient evidence that the patient’s host defenses have gained control of the infection. When the infection is resolving or has resolved, the drug treatment should be terminated. 12 , 16 , 17 A 6‑to 7‑day course would probably be appropriate for most endodontic infections. 10 According to Epstein, 18 The majority of endodontic infections resolve in 3‑7 days; thus, the 83.1% of respondents who routinely prescribe antibiotics for more than 7 days should reassess how they prescribe antibiotics.
A higher dose of antibiotics given for a shorter duration has been advocated in recent years 19 , 20. Traditionally, beta‑lactam antibiotics have been used as the first‑ line therapy for odontogenic infections. 10 In our study, amoxicillin, either alone or associated with clavulanic acid, was the most prescribed antibiotic for patients who were not allergic to penicillin. However, according to some authors, 9 , 11 the amoxicillin’s antimicrobial activity against some bacteria involved in odontogenic infections is decreasing as a result of the increasing emergence of beta‑lactamase‑ producing bacteria. Consequently, the combination of a beta‑lactam antibiotic with a beta‑lactamase inhibitor, such as amoxicillin and clavulanic acid, has been considered. 21
The association of amoxicillin with clavulanic acid is a first‑line treatment option for odontogenic infections due to its broad spectrum, low incidence of resistance, pharmacokinetic profile, tolerance and dosage. 9 , 22 , 23 In our study, amoxicillin associated with clavulanic acid was prescribed by 83.2% of respondents.However, in other studies,9,15 amoxicillin was considered as the first‑choice antibiotic in patients without penicillin allergies, followed by amoxicillin associated with clavulanic acid.
On the other hand, in the USA, amoxicillin is prescribed by only 27.5% of AAE (American Association of Endodontists) members,12 Penicillin is a narrow‑spectrum antibiotic for infections caused by aerobic gram‑negative cocci and anaerobes.12 Among the group of penicillins, penicillin VK, amoxicillin alone and amoxicillin associated with clavulanic acid have been advocated for the treatment of odontogenic infections. 19 Kuriyama et al. 24 did not find differences between their clinical evolution.
In our study, the most reported drug of choice for patients with sensitivity to penicillin was clarithromycin (34.7%). Other studies 9 , 10 reported percentages higher than 60% for clindamycin as the first‑choice antibiotic. In our study, the second antibiotic most prescribed for patients allergic to penicillin was 500‑mg azithromycin (33.7%), which is in accordance with De‑Bem et al.. 15 According to the literature, azithromycin and clarithromycin have several advantages over erythromycin, and, although azithromycin has the potential for use in endodontic infections, it is not more effective than either amoxicillin or clindamycin. 11 Due to its action spectrum and excellent penetration in the bone tissue, clindamycin is the first‑choice antibiotic for the treatment of endodontic infections in patients allergic to penicillin and cases of resistance to these drugs. 11
The majority of chronic or even acute dental infections can be successfully treated by eliminating the source of infection by pulp extirpation, drainage of abscess or tooth extraction, without the need for antibiotics.25 Thus, to justify the use of antibiotics, an infection must be persistent or systemic, i.e., cause fever, swelling, lymphadenopathy, trismus, or malaise in a healthy patient. 12
In our study, in cases of irreversible pulpitis with moderate/severe symptoms without and with an acute apical periodontitis component, 16% and 44% of the respondents prescribed antibiotics, respectively. In these cases, the pulps are still vital and there is no infection or signs of systemic involvement.
Thus, antibiotics are not indicated in either situation. 26 The findings indicate that the scientific basis for prescribing antimicrobial agents was neglected by most of the respondents.
Another study29 reported that more than 60% of respondents prescribed antibiotics for these two cases.
In cases of necrotic pulp, chronic apical periodontitis, no swelling and none or mild symptoms, in a healthy patient, there is no indication for antibiotic use, and treatment should be limited to nonsurgical root canal therapy. However, in this survey, 15.8% of the respondents prescribed antibiotics in these cases. Other studies 15 , 27 have reported prescription percentages of less than 5% in that situation; however, at the opposite end, percentages higher than 30% have been found in recent studies. 10 , 28
In situations of necrotic pulp, acute apical periodontitis, no swelling and moderate/severe symptoms, the proper treatment is debridement of the root canal space and analgesics. However, 41.1% of our sample prescribed antibiotics for this clinical situation, which is a very high percentage of inappropriate prescription. Interestingly, several other studies 9 , 10 , 12 , 28 also described higher percentages of antibiotic overuse in this situation. On the other hand, in the study by Jayadev et al., 27 solely 7.2% of the respondents prescribed antibiotics in this situation.
In asymptomatic cases of necrotic pulp and chronic apical periodontitis, and cases with sinus tracts, 45.3% of the dentists in our study still prescribed antibiotics. If there are no signs of systemic involvement, treatment of a chronic apical abscess is done similarly to other periapical pathological entities, by eliminating the etiological source present within the root canals. 11 , 29 However, if the patient is medically compromised and the sinus tract does not close within a few weeks, or the patient experiences a flare up with systemic involvement, then antibiotics would be indicated.9,12 More encouraging results were described by other studies, with percentages below 20%. 12 , 27
In the presence of necrotic pulp, acute apical periodontitis (abscess), swelling, and moderate‑to‑severe symptoms of an infection, previous studies 9 , 27 described an antibiotic prescription rate between 92% and 99%. The results of our study were comparable, at 91.6%, and appropriately so. If systemic involverev ment is considered to be present in this case, antibiotics are indicated in conjunction with debridement of the root canal space and an incision and drainage procedure.12 An exception to this trend was found in the study by Jayadev et al.,27 in which only 56% of the participants prescribed antibiotics in this case.
In cases of retreatment, according to the literature, it would only be necessary to prescribe antibiotics to treat signs and symptoms in rare situations, where the procedures of chemical‑mechanical preparation and eventual intracanal medication are not sufficient to eliminate the infectious agent 6 , 14 , 30 or in cases of immunocompromised patients. 6 , 11 , 14 , 30
The treatment of persistent pathologies should be done primarily by reviewing odontometry, recapitulating the chemical‑mechanical preparation and using eventual intracanal medication with antimicrobial activity. 6 , 11 In our study, 9.5% of the respondents prescribed antibiotics in this situation. However, a recent study31 reported a percentage of 42% of antibiotic prescriptions in the same situation.
It is important that not only the dental profession but also the general public understand the importance of restricting the use of antibiotics to those cases of true severe infection that require them.32 The use of antibiotics for minor infections, or, in some cases, in patients without infections, could be a major contributor to the world problem of antimicrobial resistance. 10 , 11 , 12
Some limitations need to be acknowledged. The reader should take into account the small sample size of our study and the possible cultural differences regarding the prescription of antibiotics. This study was carried out only in a specific city of Portugal, thus limiting the generalizability of our findings to other regions of Portugal or the entire country. All data were collected from a self‑administered questionnaire, which relied on memory and self‑reporting by the clinicians.
We have used a cross‑sectional design, which means that the results do not clarify the process and pathways leading to the choice of a specific antibiotic, or its frequency. Future qualitative and quantitative studies are clearly needed to identify the determinants of the use and misuse of antibiotics.
Conclusion
In endodontics, there is a consensus that there are situations where the use of antimicrobial agents is needed. However, in some cases, the empiricism in the moment of prescribing an antibiotic often leads health professionals to prescribe antibiotics in excess or incorrectly, thus contributing to the development of bacterial resistance and increase of ineffectiveness of the existing antibiotics in the market in the near future.
The most important conclusion in this survey is that dentists developing their activity in the Portuguese city of Viseu are over‑prescribing to irreversible pulpitis, necrotic pulps with no systemic involvement with or without sinus tracts, and persistent infections in healthy patients.
Measures should be taken to fill gaps in knowledge about the systemic antibiotic therapy by dentists. Some of them could be free mandatory annual training to update knowledge on the subject, recommendations based on scientific evidence, active awareness campaigns by the class regulator, restriction of the number of prescriptions of these drugs by regulatory bodies and measures to encourage the development of further studies aiming at evaluating antibiotic prescription patterns in other national cities.
REFERENCES
1. Cars O, Mölstad S, Melander A. Variation in antibiotic use in the European Union. Lancet. 2001;357:1851‑3.
2. Ramalhinho I, Ribeirinho M, Vieira I, Cabrita J. Consumo de antibióticos em ambulatório – Portugal 2000‑2009. Acta Med Port. 2012;25:20‑8.
3. Paiva JA, Pina E, Silva MG, Nogueira PJ, Farinha CS, Rosa MV, et al. Prevenção e Controlo de Infeções e de Resistência aos Antimicrobianos em números – 2014. Direcao‑Geral da Saúde. Lisboa: Pinto Azul, Unipessoal Lda., 2014.
4. Pallasch TJ. Global antibiotic resistance and its impact on the dental community. J Calif Dent Assoc. 2000;28:215‑33.
5. Salako NO, Rotimi VO, Adib SM, Al‑Mutawa S. Pattern of antibiotic prescription in the management of oral diseases among dentists in Kuwait. J Dent. 2004;32:503‑9.
6. Soares RG, Salles AA, Iraia LED, Limongi O. Antibioticoterapia sistêmica em endodontia: quando empregar? Stomatos. 2005;11:33‑40.
7. American Association of Endodontists. Use and Abuse of Antibiotics. ENDODONTICS: Colleagues for Excellence Newsletter: American Association of Endodontists; 2012:1‑8.
8. Al‑Haroni M. Bacterial resistance and the dental professionals’ role to halt the problem. J Dent. 2008;36:95‑103.
9. Rodriguez‑Nunez A, Cisneros‑Cabello R, Velasco‑>Ortega E, Llamas‑Carreras JM, Torres‑Lagares D, Segura‑Egea JJ. Antibiotic use by members of the Spanish Endodontic Society. J Endod. 2009;35:1198‑203.
10. Segura‑Egea JJ, Velasco‑Ortega E, Torres‑Lagares D, Velasco‑Ponferrada MC, Monsalve‑Guil L, Llamas‑Carreras JM. Pattern of antibiotic prescription in the management of endodontic infections amongst spanish oral surgeons. Int Endod J. 2010;43:342‑50.
11. Sousa ELR, Torino GG, Martins GB. Antibióticos em Endodontia – Por que, como e quando usá‑los. 1.ª ed. São Paulo: Santos, 2014.
12. Yingling NM, Byrne BE, Hartwell GR. Antibiotic Use by Members of the American Association of Endodontists in the Year 2000: Report of a National Survey. J Endod. 2002;28:396‑404.
13. Siqueira JFJ, Rôças IN. Exploiting molecular methods to explore endodontic infections: Part 2–Redefining the endodontic microbiota. J Endod. 2005;31:488‑98.
14. Oliveira JCM, Dias LA, de Uzeda M. Antibióticos sistémicos em endodontia: novos conceitos. Rev bras odontol. 2010;67:247‑54.
15. De‑Bem SHC, Nhata J, Santello LC, Bighetti RL, Cruz Filho AM. Antibiotic prescription behavior of specialists in endodontics. Dental Press Endod. 2011;1:88‑93.
16. Pallasch TJ. Pharmacokinetic principles of antimicrobial therapy. Periodontol 2000. 1996;10:5‑11.
17. Pallasch TJ. How to use antibiotics effectively. J Calif Dent Assoc. 1993;21:46‑50.
18. Epstein JB, Chong S, Le ND. A Survey of Antibiotic Use in Dentistry. J Am Dent Assoc. 2000;131:1600‑9.
19. Dar‑Odeh NS, Abu‑Hammad OA, Al‑Omiri MK, Khraisat AS, Shehabi AA. Antibiotic prescribing practices by dentists: a review. Ther Clin Risk Manag. 2010;2:301‑6.
20. Ramasamy A. A review of use of antibiotics in dentistry and recommendations for rational antibiotic usage by dentists. Int Arab J Antimicrob Agents. 2014;4:1‑15.
21. Palmer NA, Dailey YM. General dental practitioners’ experiences of a collaborative clinical audit on antibiotic prescribing: a qualitative study. Br Dent J. 2002;193:46‑9.
22. Kuriyama T, Williams DW, Yanagisawa M, et al. Antimicrobial susceptibility of 800 anaerobic isolates from patients with dentoalveolar infection to 13 oral antibiotics. Oral Microbiol Immunol. 2007;22:285‑8.
23. Seltzer S, Bender IB. Development of the Pulpodentin Complex. In: Hargreaves KM, Goodis HE, editors. Seltzer and Bender’s Dental Pulp. Chicago: Quintessence Publishing Company. 2002:13‑40.
24. Kuriyama T, Absi EG, Williams DW, Lewis MA. An outcome audit of the treatment of acute dentoalveolar infection: impact of penicillin resistance. Br Dent J. 2005;198:759‑63.
25. Al‑Haroni M, Skaug N. Knowledge of prescribing antimicrobials among Yemeni general dentists. Acta Odontol Scand. 2006;64:274‑80.
26. Smith AJ. Dentin Formation and Repair. In: Hargreaves KM, Goodis HE, editors. Seltzer and Bender’s Dental Pulp. Chicago: Quintessence Publishing Company. 2002:41‑62.
27. Jayadev M, Karunakar P, Vishwanath B, Chinmayi SS, Siddhartha P, Chaitanya B. Knowledge and Pattern of Antibiotic and Non Narcotic Analgesic Prescription for Pulpal and Periapical Pathologies‑A Survey among Dentists. J Clin Diagn Res. 2014;8:ZC10‑4.
28. Kumar KP, Kaushik M, Kumar PU, Reddy MS, Prashar N. Antibiotic prescribing habits of dental surgeons in Hyderabad city, India, for pulpal and periapical pathologies: a survey. Adv Pharmacol Sci. 2013;2013:537385.
29. Siqueira Jr JF, Rôças IN, Lopes HP. Patologias Pulpar e Perirradicular. In: Lopes HP, Siqueira Jr JF, editors. Endodontia – Biologia e técnica. 2 ed. Rio de Janeiro: Medsi. 2004:17‑77.
30. Siqueira Jr JF. Antibióticos em endodontia. In: Lopes HP, Siqueira Jr JF, editors. Endodontia – Biologia e técnica. 2 ed. Rio de Janeiro: Medsi. 2004:919‑34.
31. Nabavizadeh MR, Sahebi S, Nadian I. Antibiotic prescription for endodontic treatment: general dentist knowledge + practice in shiraz. Iran Endod J. 2011;6:5‑9.
32. Lewis MA. Why we must reduce dental prescription of antibiotics: european union antibiotic awareness day. Br Dent J. 2008;205:537‑>8.
Rita Noites
Correio eletrónico: rnoites@gmail.com
Ethical disclosures
Protection of human and animal subjects. The authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of data. The authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consent. The authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflict of interest
The authors have no conflicts of interest to declare.
Acknowledgment
The authors thanks Prof. Doutora Fiomena Capucho for her kind collaboration in reviewing this paper.
Article history:
Received 29 April 2016
Accepted 20 November 2017
Available online 17 January 2018
