
Revista Portuguesa de Estomatologia, Medicina Dentária e Cirurgia Maxilofacial
SPEMD - Revista Portuguesa de Estomatologia Medicina Dentária e Cirurgia Maxilofacial | 2023 | 64 (3) | 118-122
Clinical case
Fast recovery from Peripheral Facial Palsy in a patient with Ramsay-Hunt Syndrome using Photobiomodulation Therapy
Rápida recuperação de paralisia Facial em paciente com Síndrome Ramsay-Hunt com terapia de fotobiomodulação
a Department of Stomatology, Federal University of Paraná, Curitiba Paraná, Brazil
b Department of Biomaterials and Oral Biology, University of São Paulo, São Paulo, SP, Brazil
Article Info
Rev Port Estomatol Med Dent Cir Maxilofac
Volume - 64
Issue - 3
Clinical case
Pages - 118-122
Go to Volume
Article History
Received on 11/03/2023
Accepted on 17/07/2023
Available Online on 13/08/2023
Keywords
Clinical Case Report
Fast recovery from Peripheral Facial Palsy in a patient with Ramsay-Hunt Syndrome using Photobiomodulation Therapy
Rápida recuperação de paralisia Facial em paciente com Síndrome Ramsay-Hunt com terapia de fotobiomodulação
Bárbara Soldatelli Ballardin1 0000-0003-4385-3348
Allana Pivovar1 0000-0003-2164-7279
Gabrieli Rodrigues de Almeida Abreu1 0000-0003-1636-6990
Alyne Simões2 0000-0002-4873-1393
Melissa Rodrigues de Araujo1,* 0000-0002-2180-8223
1 Department of Stomatology, Federal University of Paraná, Curitiba- Paraná, Brazil.
2 Department of Biomaterials and Oral Biology, University of São Paulo, São Paulo – SP, Brazil.
Article history:
Received 11 March 2023
Accepted 17 July 2023
Available online 11 August 2023
Abstract
Ramsay-Hunt syndrome is an infectious disease associated with the varicella-zoster virus, and peripheral facial palsy is often one of its clinical manifestations. This case report describes a patient diagnosed with Ramsay-Hunt syndrome who developed peripheral facial palsy and was treated by photobiomodulation therapy. A 26-year-old woman developed a lack of facial mobility on the right side, asymmetry, otalgia, dysgeusia, and dry eyes, leading to the diagnosis of Ramsay-Hunt syndrome. Her peripheral facial palsy was classified as moderately severe according to the House-Brackmann scale. Acyclovir, prednisone, and physiotherapy sessions were prescribed to manage peripheral facial palsy. In the absence of a clinical response to the proposed therapies, photobiomodulation therapy was employed using the parameters: 780 nm, 100 mW, 3 J/point, and 30.48 J/cm2. After five sessions of photobiomodulation therapy, complete recovery of facial movement was observed. Photobiomodulation therapy treated peripheral facial palsy effectively, allowing a fast recovery and avoiding irreversible sequelae in a patient with Ramsay-Hunt syndrome. (Rev Port Estomatol
Keywords: Facial Paralysis, Herpes Zoster Auricularis, Laser Therapy, Photobiomodulation, Ramsay Hunt Syndrome
Resumo
A síndrome de Ramsay Hunt é uma doença infeciosa associada ao vírus varicela zoster e a paralisia facial periférica é frequentemente uma das suas manifestações clínicas. Este relato de caso descreve uma paciente com diagnóstico de síndrome de Ramsay Hunt que desenvolveu paralisia facial periférica e foi tratada com fotobiomodulação. Uma mulher de 26 anos desenvolveu falta de mobilidade facial do lado direito, assimetria, otalgia, disgeusia e olhos secos, levando ao diagnóstico de síndrome de Ramsay Hunt. A paralisia facial periférica foi classificada como moderadamente grave de acordo com a escala de House Brackmann. Foram prescritos aciclovir e prednisona, além de sessões de fisioterapia para manipulação da paralisia facial periférica. Na ausência de resposta clínica às terapias propostas, foi empregada fotobiomodulação nos parâmetros: 780 nm, 100 mW, 3 J/ponto e 30,48 J/cm2. Após cinco sessões de fotobiomodulação, foi observada uma recuperação completa do movimento facial. A fotobiomodulação foi eficaz no tratamento da paralisia facial periférica, permitindo uma rápida recuperação e evitando sequelas irreversíveis numa paciente com síndrome de Ramsay Hunt.
Palavras-chave: Paralisia Facial, Herpes Zoster Auricular, Terapia Laser, Fotobiomodulação, Síndrome de Ramsay Hunt
Introduction
Ramsay-Hunt syndrome (RHS) is a rare infectious disease associated with the varicella-zoster virus (VZV) or the human herpesvirus type 3 (HHV-3) that was described by James Ramsay Hunt in 1907.1, 2 Despite its rare incidence of five cases per 100,000 people, RHS is considered the second most common cause of peripheral facial palsy (PFP), after Bell’s palsy. 3 In addition to PFP, the symptoms and signs of the disease may include flu-like symptoms, such as inner ear dysfunction, periauricular pain, and rash, as well as herpetiform vesicles that can reach the ear pinna, external auditory canal, facial skin, tongue, hard palate, buccal mucosa, neck, and larynx.1, 3 RHS has no gender preference and generally affects individuals between 20 and 30 years old.1, 2 PFP usually occurs due to the reactivation of the VZV that remains latent in the sensory ganglion, leading to inflammation, edema, and consequente compression of the VII cranial nerve.4 - 6
Patients who develop PFP experience asymmetry and impairment of facial muscle mobility, leading to difficulty speaking and eating.7 - 9 Besides involuntary muscle contractions of the eyes, nose, forehead, and lips, excessive tearing can also be observed.9 Thus, the disease significantly affects the patient’s ability to perform daily activities, leading to a decline in quality of life.9 Clinical scales can be used to assess the evolution and severity of PFP, plan the most appropriate therapy, and establish a prognosis.9 The House-Brackmann scale,10 based on the absence or presence of facial movements, is the most employed scale.9, 10
There is still no consensus on the most appropriate treatment for PFP. The drug therapy consists mainly of prescribing corticosteroids – potent anti-inflammatory medication that reduces nerve swelling – and antivirals. Other adjuvant treatments that can be employed include electrotherapy, facial massage, acupuncture, facial expression exercises, and photobiomodulation therapy (PBMT).11 - 15
PBMT, or low-level laser therapy, is a non-invasive and painless treatment that acts on the regeneration of neurons, increases microcirculation, activates angiogenesis, and heals the damaged peripheral nerves in both sensory and motor fibers.11, 15 - 18 The absorption of photons by laser radiation is necessary to produce a photobiological response, particularly in the context of photobiomodulation. Chromophores – molecules capable of absorbing light at specific wavelengths – act as initial photoreceptors.
It has been suggested that mitochondria are highly sensitive to visible and near-infrared light in photobiomodulation. This light absorption by mitochondria can lead to various effects, such as increased production of adenosine triphosphate (ATP), enhanced DNA synthesis, modulation of reactive oxygen species (ROS) and nitric oxide species (NOS), as well as induction of transcription factors. Additionally, the absorbed energy can be transferred to other molecules, resulting in chemical reactions without significant temperature changes in the surrounding tissue.
At specific wavelengths, PBMT can activate native componentes within cells, potentially leading to alterations in biochemical reactions and cellular metabolism.15, 19 Furthermore, it modulates the inflammatory process, can be associated with other therapies, and is a widely used therapeutic modality in cases of PFP.
This case report describes a fast and complete recovery of facial palsy in Ramsay-Hunt disease treated exclusively with PBMT.
Case Report
A 26-year-old female patient sought medical care with a complaint of flu-like symptoms. In the anamnesis, she reported having been diagnosed with systemic lupus erythematosus (SLE) five years earlier, in good general condition, and controlling the disease with hydroxychloroquine (400 mg/day) since then. Due to the emerging Covid-19 pandemic and the suspicion of viral infection caused by Sars-Cov-2, a polymerase chain reaction (PCR) test was required (with a negative result) and analgesics and multivitamins were prescribed. Despite therapy and the negative Covid-19 test result, the patient presented worsening respiratory symptoms and sought emergency medical care. She was prescribed loratadine, ambroxol, analgesics, and non-steroidal anti-inflammatory drugs (NSAID) and did a second diagnostic PCR test for Sars-Cov-2 (also negative). With the progression of symptoms, the patient started to present otalgia, and at the third medical visit, an antibiotic (amoxicillin 500 mg every 8 hours for 10 days) was prescribed to replace the previously prescribed NSAID. Twenty-three days after the symptoms’ onset, she presented a lack of mobility of the muscles on the right side of the face and facial asymmetry, all clinically compatible with PFP.
Considering the patient’s signs and symptoms, the final diagnosis was RHS. The disease and its symptoms were managed with acyclovir (800 mg/5x a day), prednisone (80 mg/day for 7 days), and physiotherapy sessions. About 10 days after the end of the drug treatment and after four sessions of physiotherapy, no significant clinical response was observed. The patient had PFP for more than 17 days and reported no signs of improvement.
Due to the concern with PFP and aesthetics, the patient sought an Oral Medicine team for an evaluation. The extraoral physical examination identified the PFP associated with cranial nerve VII affecting the frontal, infraorbital, and buccal ramifications, reaching three-thirds of the face on the right side. Her PFP was classified as grade IV, which is moderately severe according to the House-Brackmann scale (Figure 1). In addition, the patient reported dysgeusia and dry eyes. PBMT was the only treatment proposed by the Oral Medicine team to manage the PFP. The parameters used in the PBMT are described in Table 1; the irradiation points are detailed in Figure 2. A significant improvement in the House-Brackmann scale was observed in the fourth PBMT session.
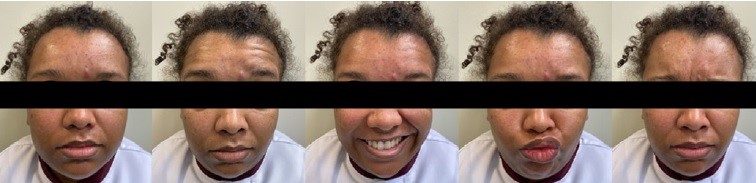
Figure 1. Facial aspect of the patient with peripheral facial palsy before photobiomodulation therapy
Table 1. Laser parameters used in the peripheral facial palsy treatment.
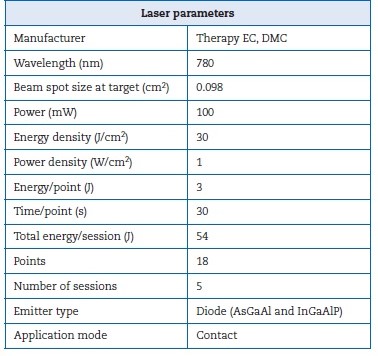

Figure 2. Photobiomodulation therapy points of the patient
After five sessions (performed twice a week for three weeks), the patient presented complete regression of the PFP and regained facial movement and expressions (Figure 3). The recovery from dysgeusia and eye dryness was gradual, accompanying the PFP improvement. The patient is still being monitored by the Oral Medicine team. Even after about 18 months of the manifestation, she has not presented new episodes or recurrence of PFP and RHS.
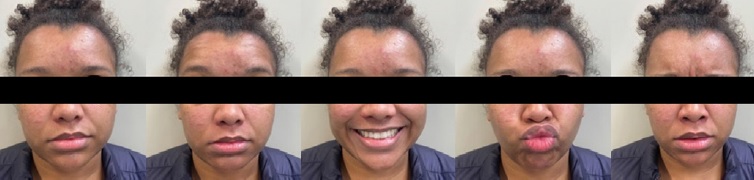
Figure 3. Facial aspect after five sessions of photobiomodulation therapy
Discussion and conclusions
Currently, there is no consensus on the most appropriate treatment for RHS. The general recommendation is a combined prescription of antivirals and corticosteroids. However, some studies argue that there is insufficient evidence regarding antiviral agents’ beneficial effects on this condition.19 In addition, corticosteroids are associated with several wellknown adverse effects.19
In 2016, Monsanto et al.1 reviewed the prognosis of facial palsy on RHS, considering the different treatments proposed in the literature. Overall, patients with RHS achieved a high rate of complete recovery of the facial nerve function (70.4%) after the different proposed treatments — mostly corticosteroids and antiviral drugs. Dosage and period of treatment varied greatly among studies. Clinical data such as age, associated metabolic diseases, impairment of the cochleovestibular or other cranial nerves, oropharynx lesions, dry eyes, and lagophthalmos must be assessed at the initial physical examination since they suggest a worse prognosis of facial palsy secondary to RHS.1
Lasting PFP that does not improve with conventional therapy is difficult to manage because it impacts the patient’s quality of life. Therefore, other therapeutic approaches, such as botulinum toxin, acupuncture, electrical stimulation, and PBMT, can be used to avoid significant sequelae.11, 15 - 18, 21 PBMT is a good therapeutic option as it is painless, has no adverse effects, and can be associated with other treatments.11, 15 - 18, 20 It acts on microcirculation, stimulating nerve regeneration and, consequently, accelerates the PFP’s recovery process.11, 15 - 18, 20 Although PBMT is associated with a shorter recovery period than drug therapies, a 3-month-long treatment may be necessary.[11]
No articles describing PBMT as a treatment for PFP in RHS were found in recent literature. On the other hand, several studies indicate PBMT as a therapy for Bell’s palsy.11, 15 - 18, Bell’s palsy is another form of PFP associated with the facial nerve that is more common and has higher recovery rates than RHS.7, 8 Despite not having a well-defined cause, Bell’s palsy may be associated with tumors, trauma, infections, or neurological and immunological diseases. Pregnancy and diabetes are also risk factors for Bell’s palsy, and the reactivation of latent herpes simplex virus is regarded as the main cause of facial nerve edema.7, 8
A systematic review study suggested that low-level laser therapy (830 nm wavelength and 80 J of total energy per session for 6 weeks) could effectively improve patients with subacute Bell’s palsy.16 As RHS is also a form of facial paralysis, PBMT can be a promising treatment. In the present study’s patient, it quickly provided good clinical results.
Some authors report that PFP’s severity and prognosis may be related to immunity and adjacent diseases of the patient.7, 8 Our patient had been diagnosed with SLE, which may be associated with the onset of the condition. However, it was not na obstacle to a good recovery using PBMT after not having shown significant improvement with drug therapy and physiotherapy.
In this case, complete and fast clinical recovery of facial movement was achieved solely using PBMT, thus avoiding irreversible sequelae.20 This result suggests that PBMT can be an effective treatment option for managing PFP associated with RHS. PFP causes asymmetry of the face. The prognosis may be fair, with complete repair in most cases. This case presented a complete PFP recovery with only five sessions of PBMT, which is a much faster response than usually reported in Bell’s palsy.
The PBMT protocol used was an effective therapy for PFP associated with RHS, and the early complete recovery impacted the patient’s quality of life.
References
1. Monsanto RC, Bittencourt AG, Neto NJB, Belike SCA, Lorenzetti FTM, Salomone R. Treatment and Prognosis of Facial Palsy on Ramsay Hunt Syndrome: Results Based on a Review of the Literature. Int Arch Otorhinolaryngol. 2016;20:394-400.
2. Boemo RL, Navarrete ML, García-Arumí AM, Copa SL, Graterol D, Scherdel EP. Ramsay Hunt syndrome: our experience. Acta Otoririnolaringol Esp. 2010;61:418-21.
3. Donati D, De Santi L, Ginanneschi F, Cerase A, Annunziata P. Successful response of non-recovering Ramsay Hunt syndrome to intravenous high dose methylprednisolone. J Neurol Sci. 2012;318:160-2.
4. de Ru JA, van Benthem PPG. Combination therapy is preferable for patients with Ramsay Hunt syndrome. Otol Neurotol. 2011;32:852-5.
5Zainine R, Sellami M. Charfeddine A, Beltaief N, Sahtout S, Besbes G. Ramsay Hunt syndrome. Eur Ann Otorhinolaryngol Head Neck Dis. 2012;129:22-5.
6. Coulson S, Croxson GR, Adams R, Oey V. Prognostic factors in herpes zoster oticus (ramsay hunt syndrome). Otol Neurotol. 2011;32:1025-30.
7. Kim SH, Jung J, Jung SY, Dong SH, Byun JY, Park MS, et al. Comparative prognosis in patients with Ramsay-Hunt syndrome and Bell’s palsy. Eur Arch Otorhinolaryngol. 2019;276:1011-6.
8. Yeo SW, Lee DH, Jun BC, Chang KH, Park YS. Analysis of prognostic factors in Bell’s palsy and Ramsay Hunt syndrome. Auris Nasus Larynx. 2007;34:159-64.
9. Fonseca KMO, Mourão AM, Motta AR, Vicente LCC: Scales of degree of facial paralysis: analysis of agrément. Braz J Otorhinolaryngol. 2015;81:288-93.
10. House JW, Brackmann DE. Facial nerve grading system. Otolaryngol Head Neck Surg. 1985;93:146-7.
11. Rodriguez CGB, Polho IB, Azevedo LH, Eduardo CP. Photobiomodulation Therapy to Treat Facial Paralysis of 8 Years: Case Report. Photobiomodul Photomed Laser Surg. 2020;38:477-80.
12. Numthavaj P, Thakkinstian A, Dejthevaporn C, Attia J. Corticosteroid and antiviral therapy for Bell’s palsy: a network meta-analysis. BMC Neurol. 2011;11:1.
13. Alptekin DO. Acupuncture and Kinesio Taping for the acute management of Bell’s palsy: a case report. Complement Ther Med. 2017;35:1-5.
14. Quinn R, Cramp F. The efficacy of electrotherapy for Bell’s palsy: a systematic review. Phys Ther Rev. 2003;8:151-64.
15. Kneebone WJ. Enhancement of nerve regeneration by therapeutic laser. Pract Pain Manag. 2010;10:70-2.
16. Javaherian M, Moghaddam BA, Tajali SB, Dabbaghipour N. Efficacy of low-level laser therapy on management of Bell’s palsy: a systematic review. Lasers Med Sci. 2020;35:1245-52.
17. Poloni MM, Marques NP, Ribeiro Junior NV, Sperandio FF, Hanemann JAC, de Carli ML. Bell’s palsy treated with photobiomodulation in an adolescent: Rare case report and review of the published literature. Int J Paediatr Dent. 2018;28:658-62.
18. Lee JH, Carpena NT, Kim SH, Lee MY, Jung JY, Choi JE. Photobiomodulation at a wavelength of 633 nm leads to faster functional recovery than 804 nm after facial nerve injury. J Biophotonics. 2021;14:e202100159.
19. Mussttaf RA, Jenkins DFL, Jha AN. Assessing the impact of low level laser therapy (LLLT) on biological systems: a review. Int J Radiat Biol. 2019;95:120-43.
20. Teixeira LJ, Valbuza JS, Prado GF. Physical therapy for Bell’s palsy (idiopathic facial paralysis). Cochrane Database Syst Rev. 2011;12:CD006283.
21. Tanganeli JPC, de Oliveira SMI, da Silva T, Fernandes KPS, Motta LJ, Bussadori SK. Complete and Fast Recovery from Idiopathic Facial Paralysis Using Laser-Photobiomodulation. Case Rep Dent. 2020;2020:9867693.
Melissa Rodrigues de Araujo
E-mail address: melissararaujo@ufpr.br
CRediT authorship contribution statement
Bárbara Soldatelli Ballardin: Data curation, Methodology, Validation, Writing – review & editing. Allana Pivovar: Data curation, Methodology, Validation, Writing – review & editing. Gabrieli Rodrigues de Almeida Abreu: Data curation, Writing. Alyne Simões: Conceptualization, Methodology, Project administration, Validation, Resources, Supervision, Writing – review & editing. Melissa Rodrigues de Araújo: Conceptualization, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing.
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical disclosures
Protection of human and animal subjects. The authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of data. he authors declare that they have followed their work center protocols on access to patient data and for its publication.
Right to privacy and informed consent. The authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Acknowledgments
The authors would like to thank the Academic Publishing Advisory Center (Centro de Assessoria de Publicação Acadêmica, CAPA – www.capa.ufpr.br) of the Federal University of Paraná (UFPR) for assistance with English language translation and developmental editing.
1646-2890/© 2023 Sociedade Portuguesa de Estomatologia e Medicina Dentária. Published by SPEMD.
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
