
Revista Portuguesa de Estomatologia, Medicina Dentária e Cirurgia Maxilofacial
Rev Port Estomatol Med Dent Cir Maxilofac | 2017 | 58 (2) | 71-78
Investigação Original
Effect of thermal cycling on the shear bond strength of different orthodontic adhesives to enamel
Efeito da ciclagem térmica sobre a resistência ao cisalhamento de diferentes adesivos ortodônticos ao esmalte
a School of Dentistry, School of Health Sciences (FCS), Universidade Fernando Pessoa (UFP), Porto, Portugal
b Center Microelectromehanical Systems (CMEMS), University of Minho, Guimarães, Portugal
c School of Dentistry (DODT), Post-Graduate Program in Dentistry (PPGO), Federal University of Santa Catarina, Florianópolis/SC, Brazil
Júlio C. M. Souza - jsouza@dem.uminho.pt
Article Info
Rev Port Estomatol Med Dent Cir Maxilofac
Volume - 58
Issue - 2
Investigação Original
Pages - 71-78
Go to Volume
Article History
Received on 29/09/2016
Accepted on 14/01/2017
Available Online on 16/05/2017
Keywords
Original research
Effect of thermal cycling on the
shear bond strength of different orthodontic adhesives to enamel
Efeito
da ciclagem termica sobre a
resistencia ao cisalhamento de diferentes adesivos ortodonticos ao esmalte
Vando L. Ribeiro-Netoa, Mónica Pinhoa, Bruno Henriquesb, Filipe S. Silvab, Sandra Gavinhaa, Júlio C. M. Souzab,c,*
a School of Dentistry, School of Health Sciences (FCS), Universidade Fernando Pessoa (UFP), Porto, Portugal
b Center Microelectromehanical Systems (CMEMS), University of Minho, Guimarães, Portugal
c School of Dentistry (DODT), Post-Graduate Program in Dentistry (PPGO), Federal University of Santa Catarina, Florianópolis/SC, Brazil
http://doi.org/10.24873/j.rpemd.2017.05.008
ABSTRACT
Objective: The aim of this study was to assess the influence of thermal cycling on the shear bond strength of three types of adhesives used for orthodontic brackets to human enamel.
Methods: Sixty six human premolars were used in this study. Two groups of dental resin composite adhesives (ED; G) and one compomer (TP) were thermal cycled from 5 up to 55 °C at 4000 cycles for 45 s in an artificial saliva solution. Then, samples were assessed by shear bond tests and inspected by optical and scanning electron microscopy. The resulting enamel surfaces were then evaluated according to the adhesive remnant index (ARI).
Results: One resin composite adhesive (group G) showed the highest shear bond strength values. Thermal cycling negatively affected the bond strength of the compomer-based adhesive although results showed clinically acceptable adhesion values after thermal fatigue for such cycles. ARI showed significant amount of residual material on the tooth surface with the adhesives that indicated strong bonding to enamel. Different adhesion mechanisms were noticed at both adhesive-enamel or adhesive-bracket interfaces.
Conclusion: The orthodontic adhesives revealed proper bond strength to enamel for clinical applications considering thermal conditions assessed in this study although the compomer-based adhesive was negatively affected by thermal cycling. The high remnant adhesive amount on enamel indicated high bond strength leading to enamel damage during debonding. (Rev Port Estomatol Med Dent Cir Maxilofac. 2017;58(2):71-78)
Keywords: Adhesive, Compomers, Fatigue, Orthodontics, Shear strength
RESUMO
Objetivo: O objetivo deste estudo foi comparar três adesivos usados na adesão de brackets ao esmalte e submetidos a testes de ciclagem térmica em saliva artificial.
Métodos: Foram testados dois grupos de adesivos baseados em resina composta (ED, G) e um grupo a base de um compomero (TP) após adesão de 72 brackets de aço inoxidável a pré-molares extraídos. Cada grupo foi dividido em dois subgrupos, sendo que um deles foi submetido a termociclagem em saliva artificial com variação de temperatura entre 5 e 55 °C. Amostras foram selecionadas para analise das interfaces por microscopia eletrónica de varrimento e os dez dentes restantes de cada um dos seis subgrupos foram submetidos a testes de resistência ao corte em uma máquina universal de testes. As superfícies de esmalte resultantes foram em seguida avaliadas segundo o Indice de Adesivo Remanescente (IAR).
Resultados: Foram detectadas diferenças estatisticamente significativas nos valores de adesão entre os 3 adesivos (p <0,05). O adesivo do grupo G, foi o que apresentou maiores valores de resistência ao corte. A termociclagem influenciou significativamente os valores de adesão do grupo TP (p <0,5). Os grupos G e TP apresentaram valores de adesão clinicamente aceitáveis também após submetidos a fadiga térmica. O IAR mostrou diferenças significativas entre os grupos de adesivos e maior quantidade de material deixado na superfície de esmalte com os adesivos que apresentaram maiores valores de adesão.
Conclusões: Um dos adesivos ortodonticos a base de resina composta apresentou adesão ao esmalte estável e aceitável para aplicações clinicas após fadiga térmica. Entretanto, a adesão do material a base de compomero diminuiu após fadiga térmica. (Rev Port Estomatol Med Dent Cir Maxilofac. 2017;58(2):71-78)
Palavras-chave: Adesivos, Compomero, Resistência ao cisalhamento, Brackets, Ciclagem térmica
Introduction
A challenge on orthodontic adhesion is to provide a strong and reliable adhesion avoiding significant destruction of the enamel surface.1 There are several types of orthodontic adhesives composed of resin composite used for allowing higher bond strengths.2 Compomers were last introduced, claiming to provide bonds as strong as those recorded for resinous adhesives.
Additionally, compomers provide the release of fluoride at low pH environment.3,3
Shear bond strength is considered clinically acceptable when values above 6 MPa are achieved.4,5 Nevertheless, bond strength values above 20 MPa are considered to be harmful to the integrity of enamel and the tooth itself.6 Previous in vitro studies have reported a large range of bond strength values for a specific adhesive. That can be explained by some variables such as: thermal cycling tests, shear test machine, direction of force applied on bracket, debonding speed, type of brackets, amount of adhesive, time elapsed between bonding and debonding, preparation of enamel surface and sample storage conditions.7 The amount of the adhesive layer has been considered as a very important factor for the adhesive bond strength on orthodontic brackets. The thickness and conversion degree of the monomers and integrity of bracket‑adhesive‑enamel assembly are factors that also influence the bonding results.8 According to some authors,8 the thickness of the adhesive layer should be lower than 250 μm.
Thermal cycling has been used in several studies for understanding the performance of the dental materials,9 and specifically orthodontic adhesive materials,10 under variations of temperature mimicking the oral cavity. The influence of thermal cycling on the adhesion properties of orthodontic adhesives to dental structures is controversial in literature.1,4,5,7‑14
Also, the comparison between resin composite adhesives and compomers has shown inconsistent results, concerning acceptable values on the shear bond strength for clinical practice.4,5
The aim of this study was to assess the influence of thermal cycling on the bond strength of three different orthodontic adhesives to enamel as well as to compare the bond strength values between two resin composites and a compomer‑based adhesive. The null hypothesis of this work was that there were no significant differences in shear bond strength values among the orthodontic adhesive after thermal cycling tests.
Materials and methods
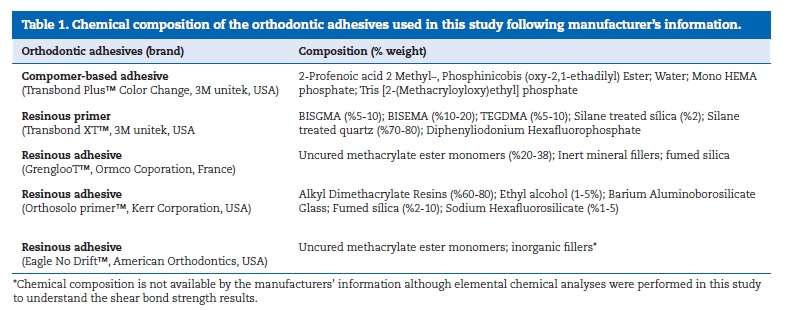
Sixty‑six human premolars with undetermined time of extraction were cleaned from any residual soft tissues and then immersed in chloramine solution at 4 oC over a period of 7 days. Then, teeth were stored in distilled water at a temperature of 4 °C during 7 days for hydration prior to bonding procedures.9,10 Teeth were processed following the technical specification ISO/TS 106 SC 11405:2003. After that, the teeth were cleaned with water and green stone at low speed and then rinsed with water spray and air‑dried under oil‑free airstream for 3 s.9,10 The bonding surface was etched using 37% phosphoric acid (Octacid, Clarben S.A., pH < 2 at 20 °C) for 30s. Then, teeth were washed during 60 s and gently dried under airstream for 3 s. Teeth were divided correspondingly for bonding considering three orthodontic adhesives: TP group: TransbondTM Plus Color Change/TransbondTM XT Primer (3M unitek, USA); G group: GrenglooTM/Ortho SoloTM Primer, (Ormco Corporation, FRA); ED group: Eagle No DriftTM (American Orthodontics, USA). The chemical composition of the orthodontic adhesives is shown in Table 1.
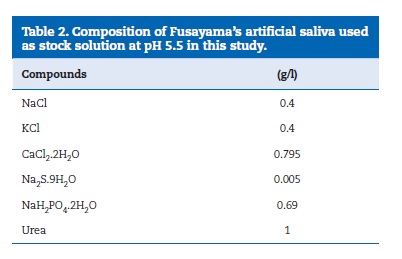
Sixty‑six stainless steel brackets (Master Series, American Orthodontics, USA) were bonded to enamel surface. The bracket’s bonding surface area was calculated as being 10.3 mm2, using a 3D virtual model of the brackets by software (SolidWorks, USA). One blinded and well‑trained operator performed the bonding procedure of the brackets to the enamel surfaces along the axis of the crown according to the manufacturer instructions. In all groups, excess composite material was removed with an explorer without disturbing bracket placement. Adhesives were light‑ cured by continuous mode at 420‑490 nm using a LD‑105 curing device (1000 mW/cm2, Monitex, China) for 30 s: on mesial and distal for 10 s each, over the bracker for 10 s. Afterwards, half of the samples were randomly chosen for thermal cycling tests (n =10). The thermal cycling tests were performed in Fusayama’s artificial saliva solution (Table 2) at a temperature ranging from 5 up to 55 °C12 for 4000 cycles, according to the ISO TR11450 (1994). Each cycle time corresponded to 45 s distributed in 15 s of dwell time in each bath and 15 s of transfer time.
One sample from each six subgroup was randomly chosen for scanning electron microscopy (SEM) observation of the interface. The remaining sixty were positioned with the bracket base placed horizontally and then stabilized using a resin composite. For the shear tests, all teeth were embedded in PVC molds within self‑ curing acrylic resin to be attached on a metallic holding device, that only the coronal region was exposed. Debonding was performed by axial loading on the bracket wing, parallel to the bracket base, using an universal testing machine (Instron 8874, 25kN; Instron Corp., Norwood, Massachusetts, USA) at a crosshead speed of 0.5 mm/min (n = 10).4,5,9‑16
Six samples (teeth with bracket) were randomly chosen for microscopic observation, corresponding to one of each type of adhesive subjected or not to thermal clycing tests. After debonding, each sample (=10) was inspected by optical microscopy (Axiotech, Carl Zeiss, USA) to evaluate the fracture pathways at magnification ranging from x10 up tp x500. The residual adhesive on the tooth was evaluated using the adhesive remnant index (ARI) for 4 scores.1,7
For scanning electron microscopy (SEM), samples were mounted in acrylic resin and cross‑sectioned perpendicular to the bracket‑adhesive interface plan. The cross‑sectioned samples were wet ground on silicon carbide (SiC) papers down to 2500 mesh and polished using 1 μm diamond slurry. The teeth‑adhesive‑bracket region of each sample was then inspected by SEM on secondary (SE) and backscattered (BSE) electron mode at magnification ranging from x100 up tp x2000 at 15 kV.
The surfaces were previously coated with a gold film. The inspection of bracket bases after debonding was also produced.
Data were statistically analyzed by two‑way ANOVA using SPSSR v.21.0 (SPSS Inc., IL. Chicago, USA). The level of statistical significance was set at 0.05. The two‑way ANOVA was used to determine differences between adhesive groups subjected or not on thermal fatigue and within groups for the same adhesive (p<0.05). Then post hoc tests were applied to evaluate the significant differences in the bond strength of the adhesives.
T‑test for independent samples was performed to discover which adhesives were significantly influenced by thermal fatigue.
Regarding ARI tests, Kruskal‑Wallis test was used to verify the existence of significant differences between at least two adhesives (p<0.05), followed by Mann‑Whitney tests aiming at understanding which adhesives differed significantly.
Results
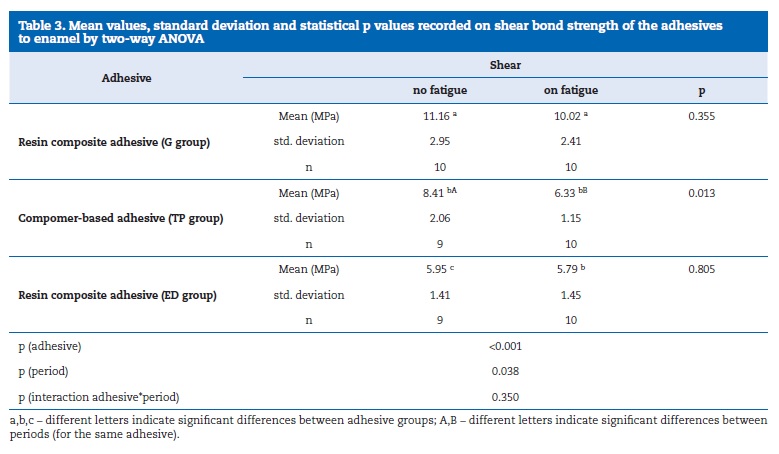
The bond strength results are shown in Table 3. Group G showed the highest bond strength results while the lowest values were recorded for group ED, regardless thermal cycling. Groups G and TP showed acceptable values for clinical practice. Significant differences were found between adhesives and between periods for the same adhesive (p<0.05). The interaction adhesive/thermal cycling was not statistically significant (ANOVA, p=0.350).
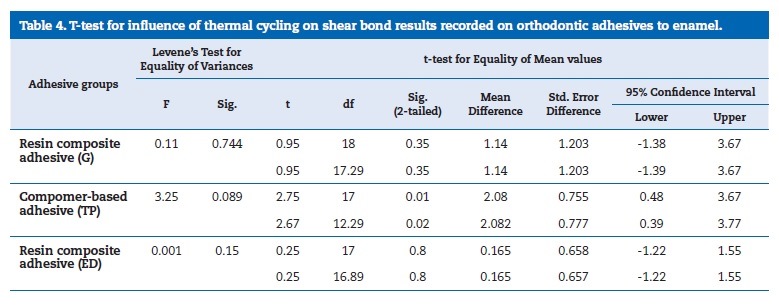
Post hoc tests Tukey HSD and LSD showed significantly higher bond strength results for G and TP groups, without effect of the thermal cycling. On thermal cycling, only the difference between G and TP was statistically significant. The t‑test
(Table 4) showed that only TP compomer was significantly affected by thermal cycling (p=0.013).
The failure mode exhibited by the adhesives scored as according to the ARI is shown in Table 5. ARI scores noted discarding the effect of thermal cycling were mostly 1 and 2 although those increased to 2 and 3 considering the effect of fatigue. According to Mann‑Whitney Test, significant differences were found on ARI values between the non‑thermal cycled samples for ED group and the other two adhesives (p<0.05). On thermal cycling, the significant differences in ARI values were only found between TP and ED. Some teeth bonded with G and TP adhesives revealed fracture on enamel after shear bond strength tests and they were removed from ARI assessment.
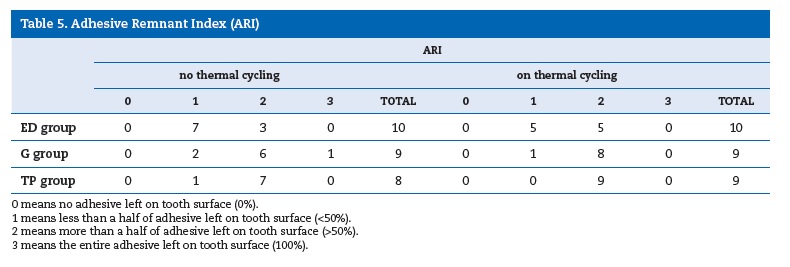
The shear bond strength plotted against the percentage of adhesive remnants on the teeth surface subjected to shear bond tests is shown in Figure 1. Therefore, the amount of adhesive remnants tend to increase for thermal cycled specimens, relative to non‑thermal cycled ones (Table 5). This is more evidenced for G and ED adhesives, which exhibited high shear bond strength values.
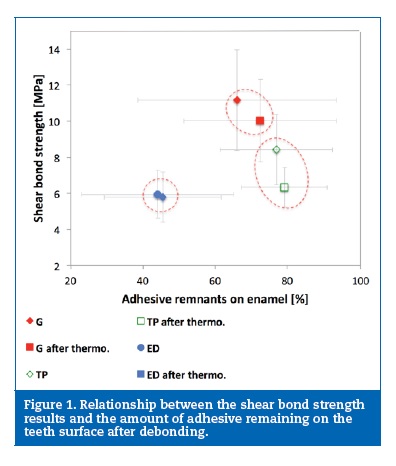
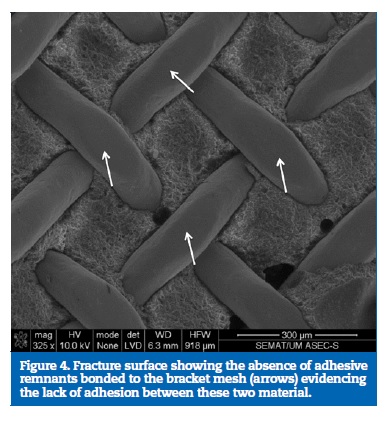
A cross‑section overview of the tooth‑adhesive‑bracket region is shown in Figure 2. A careful inspection of this site revealed the lack of adhesion between the brackets and the adhesives, evidenced by the presence of interfacial gaps between the two materials in all samples examined (Figure 3). This fact was further emphasized in Figure 4 that showed the fracture surface with total absence of adhesive remnants bonded to the metallic mesh of the bracket base. ED group presented its fracture interface between adhesive and enamel surface (Figure 5).
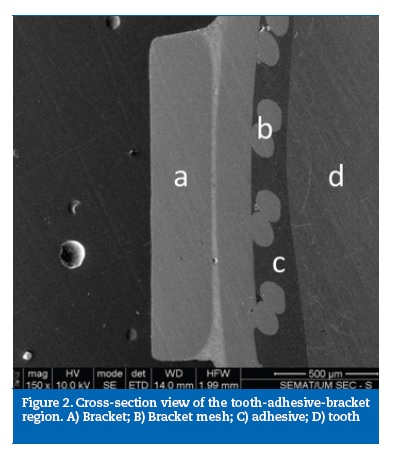

Discussion
This paper revealed novel information on the behavior of orthodontic adhesives subjected or not to thermal cycling. Results showed a detrimental effect of the thermal cycling on the bond strength of the test adhesives to enamel, with a statistically significant decrease being only recorded for the compomer group. Such results supported the rejection of the null hypothesis of the present study and therefore they are in agreement with the results found in literature.10,17
Considering shear bond tests on adhesive groups free of thermal cycling, G group was the resin composite adhesive that exhibited the highest bond strength at 11.16 ± 2.95 MPa.
TP group reached values of 8.41 ± 2.06 MPa that was similar to shear bond values found in previous studies.13,14,18 However, shear bond strength values exceeding 15 MPa were also reported in several previous studies.4,15,16 In this study, TP compomer achieved clinical acceptable values (above 6 MPa), in agreement with some other recent studies.5,13-16,18,19 However, significant differences were recorded for the test adhesives, mainly for G and ED, suggesting an influence of the chemical composition and properties of the adhesive on their bond strength to the teeth surface.5,13-16,18
Despite the decrease in bond strength for TP compomer subjected to thermal fatigue in this study, that is still considered clinically acceptable considering values recorded above 6 MPa in previous studies. Additionally, the benefits of such orthodontic adhesives considering fluoride release and prevention of enamel demineralization supported by several studies3,220 should also be considered on the treatment of caries susceptible patients. Previous studies have reported different results considering the effect of thermal cycling on shear bond strength for such compomer‑based adhesive.14,21 For instance, a previous study revealed stable shear bond strength of orthodontic adhesives to enamel after thermal cycling between 5 and 60 oC although samples were tested only for 500 cycles.14
Another previous study reported a decrease in shear bond strength after 1000 thermal cycles between 4 and 60 oC. Concerning the performance of the materials in oral cavity, additional tests must be performed for longer period of thermal cycling to simulate the orthodontic treatment time.
ARI showed most adhesive remnants on enamel surface on the two adhesives that displayed higher and clinically acceptable shear bonding values on thermal cycling. However different findings were noted in other studies involving G and TP group5,16,17,20,21 whereas different amounts of adhesive were recorded on the enamel surface after bond strength tests. Group ED achieved score 1 without effect of thermal cycling. On thermal fatigue, equal number of samples with score 1 and 2 were achieved. No study using ED adhesive was found in literature. ARI results showed a tendency, despite not statistically significant, to high shear bond strength being related to higher amounts of adhesive remnants on the enamel surface. This is in agreement with the findings of some others studies.4,5 Some previous studies concluded that the increase in bond strength of adhesives resulted in higher amount of adhesive remnants on enamel surface1,7 while groups with lower bonding strength showed more adhesive on bracket base.7 The balance between bond strength of the adhesive to enamel and enamel damage is the main key to obtain successful results in an orthodontic treatment regarding the procedure of bracket bonding.21
Also, results revealed a tendency for thermal cycled specimens to exhibit lower bond strength values together with higher percentage of adhesive remnants on the enamel surface.
This might be explained by the detrimental effect of the thermal cycling on the interface between adhesive and the bracket base. In fact, the microgaps existing at that interface allowed the penetration of the artificial saliva at these locations and to accelerate up the adhesive’s deterioration.7
Higher prevalence of cohesive fractures was thus expected to occur. On the interface microstructure, the adhesive thickness remained between 200 and 250 μm according to SEM evaluation. That is considered to provide the proper bonding results.8,15 However, the thickness of the adhesive is not the same considering variations related to the operator technique, materials’ properties and geometry of the teeth surfaces.8,15,21 It is noteworthy to highlight the lack of connection between the bracket base and the adhesive, when compared to the good adhesive‑enamel interface. The negative effects of these microgaps, as discussed above, can be addressed by a surface treatment on the bracket base allowing the establishment of a satisfactory bond between the two materials.
Conclusions
Within the limitations of this study, the following conclusions can be drawn:
– A resin composite adhesive showed a high bond strength value at about 11 MPa that is proper for clinical application. The compomer‑based adhesive was the only adhesive significantly affected by the thermal cycling.
– The orthodontic adhesives tested in this study revealed high adhesion strength that resulted in high amount of adhesive remnants on the enamel surface, after debonding.
– The interface between the adhesive and the enamel showed better integrity than that between the adhesive and the bracket.
References
1. Su MZ, Lai EH, Chang JZ, Chen HJ, Chang FH, Chiang, YC, Lin CP. Effect of simulated debracketing on enamel damage. J Formos Med Assoc. 2012;11:560‑6.
2. Ewoldsen N, Demke R S. A review of orthodontic cements and adhesives. Amer J Orthod Dentofac Orthop. 2001;20:45‑8.
3. Chin MY, Sandham A, Rumachik EN, Ruben JL, Huysmans MC. Fluoride release and cariostatic potencial of orthodontic adhesives with and without daily fluoride rinsing. Am J Orthod Dentofacial Orthop. 2009;136:547‑53.
4. Haydar B, Sarikaya S, Cehreli ZC. Comparison of shear bond strength of three bonding agents with metal and ceramic brackets. Angle Orthod. 1999;69:457‑62.
5. Ekhlassi S, English JD, Ontiveros JC, Powers JM, Bussa HI, Frey GN, Colville CD, Ellis RK. Bond strength comparison of color‑change adhesives for orthodontic bonding using a self‑etching primer. Clin Cosmet Investig Dent. 2011;3:39‑44.
6. Ludwig B, Glasl B. Materials. In Ludwig B, Bister D, Baumgaertel S. Self‑ligating Brackets in Orthodontics: Current Concepts and Techniques. Thieme, Stuttgart. 2012, 10‑33.
7. Baherimoghadam T, Akbarian S, Rasouli R, Naseri N. Evaluation of enamel damages following orthodontic bracket debonding in fluorosed teeth bonded with adhesion promoter. Eur J Dent. 2016;10:193‑8.
8. Arici S, Caniklioglu CM, Arici N, Ozer M, Oguz B. Adhesive thickness effects on the bond strength of a light‑cured resin‑modified glass ionomer cement. Angle Orthod. 2005;75:254‑9.
9. Gomes P, Portugal J, Jardim L. Effect of high‑powered LED‑curing exposure time on orthodontic bracket shear bond strength. Rev Port Estomatol Med Dent Cir Maxilofac. 2014;55:78‑82.
10. Mendes M, Portugal J, Arantes‑Oliveira S, Mesquita P. Shear bond strength of orthodontic brackets to fluorosed enamel. Rev Port Estomatol Med Dent Cir Maxilofac. 2014;55:73‑7.
12. Alkis H, Turkkahraman H, Adanir N. Microleakage under orthodontic brackets bonded with different adhesive systems. Eur J Dent. 2015;9:117‑21.
12. Henriques B, Goncalves S, Soares D, Silva FS. Shear bond strength comparison between conventional porcelain fused to metal and new functionally graded dental restorations after thermal‑mechanical cycling. J Mech Behav Biomed Mater. 2012;13:194‑205.
13. Yuasa T, Iijima M, Ito S, Muguruma T, Saito T, Mizoguchi I. Effects of long‑ term storage and thermocycling on bond strength of two self‑etching primer adhesive systems. Eur J Orthod. 2010;32:285‑90.
14. Vinagre AR, Messias AL, Gomes MA, Costa AL, Ramos JC. Effect of time on shear bond strength of four orthodontic adhesive systems. Rev Port Estomatol Med Dent Cir Maxilofac. 2014;55:142‑51.
15. Protasio MF, Frota PHD, Costa JF, Carneiro KK, Bauer J. Effects of application mode of self‑etching primer on shear bond strength of orthodontic brackets. Rev Port Estomatol Med Dent Cir Maxilofac. 2016;57:9‑13.
16. Turkkahraman H, Adanir N, Gungor AY, Alkis H. In vitro evaluation of shear bond strengths of colour change adhesives. Eur J Orthod. 2010; 32:571‑4.
17. Van Landuyt KL, Snauwaert J, De Munck J, Peumans M, Yoshida Y, Poitevin A, Coutinho E, Suzuki K, Lambrechts P, Van Meerbeek B. Systematic review of the chemical composition of contemporary dental adhesives. Biomater. 2007;28:3757‑85.
18. Montasser MA. Effect of applying a sustained force during bonding orthodontic brackets on the adhesive layer and on shear bond strength. Eur J Orthod. 2011;33:402‑6.
19. Izadi MI, Sherriff M, Cobourne MT. A comparative investigation into relative bond strengths of Damon3, Damon3MX, and APC II brackets using different primer and adhesive combinations. Eur J Orthod. 2012;34:778‑82.
20. Behnan SM, Arruda AO, Gonzalez‑Cabezas C, Sohn W, Peters MC. In‑vitro evaluation of various treatments to prevent demineralization next to orthodontic brackets. Am J Orthod Dentofacial Orthop 2010;138:712.e1‑7.
21. Hama T, Namura Y, Nishio Y, Yoneyyama T, Shmizu. Effect of orthodontic adhesive thickness on for required by debonding pliers. J Oral Scie. 2014;56:185‑90.
22. Endo T, Ishida R, Komatsuzaki A, Sanpei S, Tanaka S, Sekimoto T. Effects of long‑term repeated topical fluoride applications and shear bond strengths of orthodontic brackets. Eur J Dent. 2014;8:431‑6.
Julio C. M. Souza
E-mail address: jsouza@dem.uminho.pt
Ethical disclosures
Protection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of data. The authors declare that no patient data appear in this article.
Right to privacy and informed consent. The authors declare that no patient data appear in this article.
Conflicts of interest
The authors have no conflicts of interest to declare.
Aknowledgments
Authors acknowledge Fundacao para Ciencia e Tecnologia (FCT) (NORTE-01-0145-FEDER-000018 – HAMaBICo) for the financial support. Also, authors a thank you to Mr. Helder Martins and to Orthosmile ltda for providing the materials tested in this study.
Article history:
Received 29 September 2016
Accepted 14 January 2017
Available online 16 May 2017
