
Revista Portuguesa de Estomatologia, Medicina Dentária e Cirurgia Maxilofacial
SPEMD | 2019 | 60 (3) | 118-124
Original research
Oral manifestations in patients with different oral health behaviors submitted to chemotherapy: a preliminary study
Manifestações orais em pacientes com diferentes comportamentos de saúde oral submetidos a quimioterapia: estudo preliminar
a Faculdade de Medicina Dentária, Universidade de Lisboa, Lisbon, Portugal
Jéssica Gilberto Santilal - jessica_gilberto@hotmail.com
Article Info
Rev Port Estomatol Med Dent Cir Maxilofac
Volume - 60
Issue - 3
Original research
Pages - 118-124
Go to Volume
Article History
Received on 17/04/2019
Accepted on 12/10/2019
Available Online on 29/10/2019
Keywords
Original research
Oral manifestations in patients with different oral health behaviors submitted to chemotherapy: a preliminary study
Manifestacoes orais em pacientes com diferentes comportamentos de saude oral submetidos a quimioterapia: estudo preliminar
Jéssica Gilberto Santilal*, Sandra Ribeiro Graça
Faculdade de Medicina Dentária, Universidade de Lisboa, Lisbon, Portugal
http://doi.org/10.24873/j.rpemd.2019.10.460
Abstract
Objective:This observational study aims to evaluate oral manifestations and symptoms during chemotherapy in 49 adult patients with onco-hematologic pathology, and to investigate whether oral health habits influence the experience of signs and symptoms in the oral cavity and the quality of life of these patients.
Methods:The study was carried out in the outpatient clinic of the Hematology Department and the Myeloma Clinic of the Instituto Portugues de Oncologia Francisco Gentil de Lisboa. Data were collected by a questionnaire and oral examination. Mann-Whitney and Kruskal-Wallis tests were used to verify possible associations between the number of oral manifestations and the cycles and duration of chemotherapy, frequency of dental appointments and quality of life.
Results:The results showed that 89.8% of the patients reported oral symptoms, of which the most frequent were xerostomia (71.7%) and dysgeusia (47.8%). About a quarter of the patients reported a worse quality of life during chemotherapy (p=0.001), which was associated with a higher number of oral manifestations (p=0.002). No associations were found between the symptoms and the number of manifestations and the use of dental services before or during chemotherapy, oral hygiene habits or the number of chemotherapy cycles. However, the duration of chemotherapy influenced the number of oral manifestations (p=0.014).
Conclusions:This study demonstrated that chemotherapy influences oral health negatively and compromises the quality of life. The dental-medical follow-up of these patients is fundamental to minimize the consequences of this therapy.
Keywords: Chemotherapy, Oral health, Oral hygiene habits, Oral manifestations, Quality of life
Resumo
Objetivo:Este estudo observacional, tem como objetivo avaliar as manifestações orais e os sintomas durante a quimioterapia em 49 pacientes adultos com patologia onco-hematologica e investigar se os hábitos de saúde oral influenciam a experiencia de manifestações e sinais na cavidade oral e a qualidade de vida destes pacientes.
Métodos:O estudo decorreu no Instituto Português de Oncologia Francisco Gentil de Lisboa, no Hospital de Dia, em pacientes dos Serviço de Hematologia e Clinica de Mieloma. Os dados foram recolhidos por questionário e por exame clinico oral. Foram utilizados testes Mann Whitney e Kruskal Wallis para verificar possíveis associações entre o número de manifestações orais com ciclos e duração da quimioterapia, frequência de consultas de medicina dentária e qualidade de vida.
Resultados:Os resultados obtidos demonstram que 89,8% dos sintomas orais sendo os mais frequentes a xerostomia (71,7%) e disgeusia (47,8%). Cerca de um quarto dos pacientes reportaram pior qualidade de vida durante a CT (p=0,001) com associação a um maior numero de manifestações orais (p=0,002) não foram encontradas associações entre o uso de serviços dentários antes ou durante a quimioterapia, hábitos de higiene oral e numero de ciclos da quimioterapia. Contudo, a duração da quimioterapia influenciou negativamente o número de manifestações orais (p=0,014).
Conclusões:Este estudo demonstrou que a quimioterapia influencia a saúde oral e compromete a qualidade de vida. O acompanhamento medico-dentário destes pacientes e fundamental para minimizar as consequências desta terapêutica.
Palavras-chave: Quimioterapia, Saúde oral, Hábitos de saúde oral, Manifestações orais, Qualidade de vida
Introduction
Nowadays, cancer is one of the most significant health problems. Chemotherapy (CT) is one of the most used contemporary treatments, by at least 70% of patients.1 This treatment is based on destroying or inhibiting abnormal cell growth without any differentiation between normal and carcinogenic cells.2 CT is usually toxic to cells with high turn-over, which entails not only cancer cells but also bone marrow and the gastrointestinal tract mucosa, including the oral mucosa. One study reported the development of oral complications in 40% of patients submitted to CT.1
It is known that CT-related oral complications can result from direct or indirect mechanisms.3, 4 The direct effects result from the toxicity of the drug on oral structures and include mucositis, dysgeusia (usually temporary), neurotoxicity (a toothache-like severe pain not associated with a local pathology), xerostomia and salivary gland dysfunction. The indirect side effects are based on myelosuppression and immunosuppression and may result in a propensity for bacterial, fungal and viral infections.5 Sudden oral bleeding can also occur associated with thrombocytopenia.1 It is evident in the literature that oral manifestations can be severe and serious, affecting the success of CT treatment.6, 7 These oral complications can threat the patient’s survival by compromising the discontinuation or requiring a change in the CT protocol.8 The CT-related manifestations in the oral cavity also compromise oncology patients’ quality of life, mainly their physical and social functions.2, 4, 5
Several factors can influence the presence and severity of oral complications, such as the patient’s age, the cancer type and location, the nutritional status, the dental and periodontal health, and the oral hygiene status before and after treatment.4, 9 Also, the dosage and frequency of use of the chemotherapeutic agents can influence the severity of oral complications.7 Currently, CT is usually given in cycles with varying intensity depending on the disease and, usually, on an outpatient basis. There are specific protocols that vary in duration (minutes, hours or days), frequency (weekly, bi-weekly or monthly) and number of cycles (depending on the type of cancer and the phase of cancer treatment and follow-up).10 CT protocols have been changing in the past years to minimize side effects, with the use of new drugs and techniques and also fewer drugs, which reduces chemotherapeutic side effects, thus allowing more time for normal cells to recover from damage.11
Since the oral cavity suffers so many side effects of CT, it is relevant to inquire whether the oral health routines before and during CT affect oral health, CT consequences and quality of life in these patients. As reported in the literature, careful oral hygiene is effective in reducing oral complications in patients submitted to CT.3 In the same way, including an oral health professional in the onco-hematology team can reduce oral complications, as well as increase the comfort and quality of life of these patients before, during and after CT.6
At the time of this study, the authors found some studies about CT-related oral complications and symptoms in pediatric populations.3, 12, 13 However, studies about the prevalence of CT oral manifestations in adult populations, as well as the patient’s associated experience, were scarce. There were also some CT studies associated with other oncology therapies, like radiotherapy.14
This study aimed to investigate oral manifestations and symptoms during CT in a sample of onco-hematology patients, and whether oral health routines influence the patient’s experience of oral manifestations and symptoms.
Material and Methods
This observational study was conducted in November and December 2017 in an adult population submitted to CT at the Francisco Gentil Portuguese Institute of Oncology – Lisbon Center (Instituto Portugues de Oncologia Francisco Gentil de Lisboa – IPOFGL). Patients were recruited from the outpatient clinic of two departments, which gave permission: Hematology Department and Myeloma Clinic (Servico de Hematologia e Clinica de Mieloma). To be eligible to participate, patients could not have conditions that affect the logic, such as dementia or other incapacities that would impede them from answering the questionnaire with consciousness and responsibility.
Patients that had been submitted to major oral surgery and/or head and neck radiotherapy were also excluded because these could compromise the interpretation of CT effects independently.
For data collection, a questionnaire based on the literature and the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire – Head and Neck module (EORTC QLQ–H&N35) was used.15 The questionnaire had three sections. The first was about the oncologic pathology (diagnosis, location of the pathology, treatment modality and medication).
The second section was about daily oral hygiene routine before and after CT. The last section was the self-reported changes in the oral cavity during CT as well as the oral health-related quality of life. Lastly, the participant’s sociodemographic data were also recorded. An oral examination was performed to evaluate the decayed, missing and filled teeth (DMFT)16 index, the simplified oral hygiene index (OHI-s),17 and the presence/absence of oral changes (hyperplasia, recessions, mobility, mucositis, xerostomia, ulcers/sores, infection, sensibility, halitosis, loss of appetite, Candidiasis and alike, white/red lesions, herpes, dysgeusia, gingival changes, mouth soreness, tooth pain, difficulty in speech and feeding, and temporomandibular joint pain). The questionnaire and the oral examination took part during one session of CT treatment in an outpatient hospital of IPOFGL.
The Council of Research, the Ethics Commission and the Administration Board of IPOFG approved the study protocol (Ref UIC/1099). Each potential participant was invited, and signed informed consent was obtained. Data were analyzed using the software Statistical Package for the Social Sciences (SPSSR, version 25). Descriptive statistics were used to illustrate the changes felt and observed during and after CT. The Mann-Whitney and the Kruskal-Wallis tests were used to verifying potential associations between the number of oral manifestations and the cycles and duration of CT, the frequency of dental appointments and the quality of life. Changes in quality of life were assessed using a sign rank test.
Results
The final sample consisted of 49 patients treated in an outpatient hospital, with a mean age of 53.6 years (±19.2 [18-84]).
The majority of patients were male (53.1%), had primary education or less (53.1%) and had intermediate professional qualifications (57.1%).
The most observed oncologic pathology was lymphoma (67.3%), followed by leukemia (26.5%); there were a few cases of myeloma (4.1%). At the time, 48 (98%) patients had been doing CT for more than three months (53.1%). In all cases, chemotherapeutic agents were delivered intravenously.
The number of CT cycles varied between one (14.3%) and six or more (34.6%), with almost two-thirds of patients having had less than six cycles (65.3%). Besides CT, 11 (22.4%) patients had also had radiotherapy and/or surgery in locations other than the head and neck. Additionally, all patients were under other medications besides CT for other systemic conditions, of which cardiovascular drugs were the most frequent (60.7%).
Metabolic and digestive system drugs were the second most frequent drugs (57.1%). Some patients were also under antibiotics and nervous system drugs (14.3% and 10.7%, respectively).
All patients brushed their teeth daily, with 91.8% of the patients doing it at least twice a day. Interdental means of plaque removal were used by a quarter (26.5%) of the sample, with dental floss being the most used (18.4%). Mouth rinses were commonly used by 71.4% of the patients.
Almost 70% of patients received instructions on daily oral hygiene, and the most frequent instruction was rinsing (67.3%) with a non-alcoholic solution (69.4%). Diet recommendations were less frequently reported (24.5%), and a soft diet (75%) was the most common recommendation. No patient reported being recommended a cold diet, a non-spicy diet, an acid diet or tobacco and alcohol avoidance.
Eighteen patients (36.7%) made changes to their oral hygiene routine, and, for 94.4% of those, the change was to intensify toothbrushing or rinsing frequencies. One patient (2.0%) reported that he started brushing less often. Regarding diet, 14.3% of the patients did not make any change during CT.
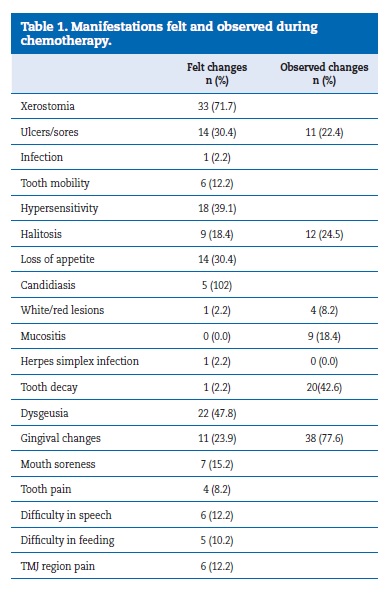
Most patients (89.8%) felt changes in their oral cavity during CT. The most common change was xerostomia (71.7%), followed by dysgeusia (47.8%), dental hypersensitivity (39.1%) and mouth ulcers and loss of appetite (30.4%). Pain and function difficulties were reported by 13 patients (26.5%), and mouth soreness (15.2%), TMJ pain (12.2%), difficulties in speech (12.2%), difficulties in feeding (10.2%) and tooth pain (8.2%) were also mentioned. In the intraoral examination, gingival changes (inflammation, hemorrhage, and hyperplasia) were the most common oral manifestation (77.6%), followed by dental caries (42.6%) and halitosis (24.5%). Mucositis was observed in nine patients (18.4%) (Table 1).
When asked if their quality of life was affected during CT, most patients reported not at all (24.5%) or a little (38.8%), whereas 36.7% reported very much. Regarding their self-assessed oral health, 51% of the patients rated their oral health before CT as good or very good and 4.1% as bad, while 40.8% of the patients rated their oral health during CT as good or very good and 18.4% as bad. Eleven patients (22.4%) rated their quality of life as worst during CT (p=0.001; sign rank test).
The utilization of dental services before starting CT was reported by 53.1% of the patients compared to 10.2% during CT. Concerning patients that had already finished previous cycles of CT, 53.8% of them had visited the dentist after the completion of the cycle.
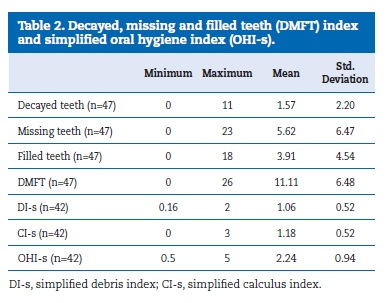
Forty-seven (95.9%) patients had natural teeth, and the mean number of teeth was 21.1 (± 8.13). Thirteen (26.5%) patients wore dental prostheses. The DMFT was 11.11 (± 6.48), with the missing component contributing the most (5.62 ± 6.47). The OHI-s mean, which results from the sum of the debris and calculus indexes, was 2.24 (± 0.94), representing a fair oral hygiene score (Table 2).
The intraoral and periodontal exam revealed the presence of tooth recessions (73.5%), tooth mobility (12.2%) and gingival inflammation (77.6%). Halitosis was observed in 12 (24.5%) patients.
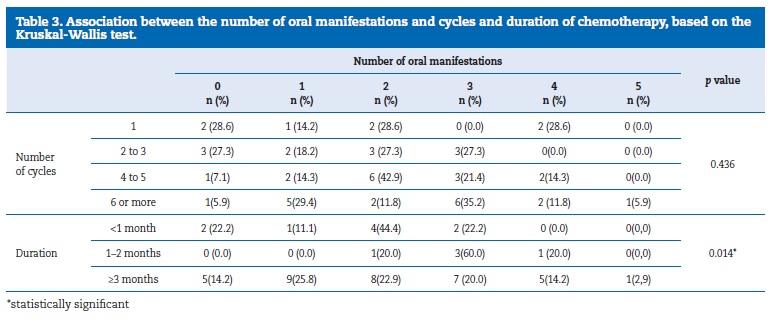
The number of oral manifestations was not associated with the number of CT cycles (p=0.436) but was associated with the duration of the CT treatment (p= 0.014) (Table 3).
When asked if their quality of life was affected during CT, most patients reported not at all (24.5%) or a little (38.8%), whereas 36.7% reported very much. Regarding their self-assessed oral health, 51% of the patients rated their oral health before CT as good or very good and 4.1% as bad, while 40.8% of the patients rated their oral health during CT as good or very good and 18.4% as bad. Eleven patients (22.4%) rated their quality of life as worst during CT (p=0.001; sign rank test).
The utilization of dental services before starting CT was reported by 53.1% of the patients compared to 10.2% during CT. Concerning patients that had already finished previous cycles of CT, 53.8% of them had visited the dentist after the completion of the cycle.
Forty-seven (95.9%) patients had natural teeth, and the mean number of teeth was 21.1 (± 8.13). Thirteen (26.5%) patients wore dental prostheses. The DMFT was 11.11 (± 6.48), with the missing component contributing the most (5.62 ± 6.47). The OHI-s mean, which results from the sum of the debris and calculus indexes, was 2.24 (± 0.94), representing a fair oral hygiene score (Table 2).
The intraoral and periodontal exam revealed the presence of tooth recessions (73.5%), tooth mobility (12.2%) and gingival inflammation (77.6%). Halitosis was observed in 12 (24.5%) patients.
The number of oral manifestations was not associated with the number of CT cycles (p=0.436) but was associated with the duration of the CT treatment (p= 0.014) (Table 3).
The quality of life during CT was significantly associated with the number of oral manifestations (p=0.002) (Table 4). The value of the Pearson ρ was 0.521 (p<0.001), indicating a moderate correlation between these two variables, which means that patients whose quality of life was more affected had a higher number of oral manifestations.

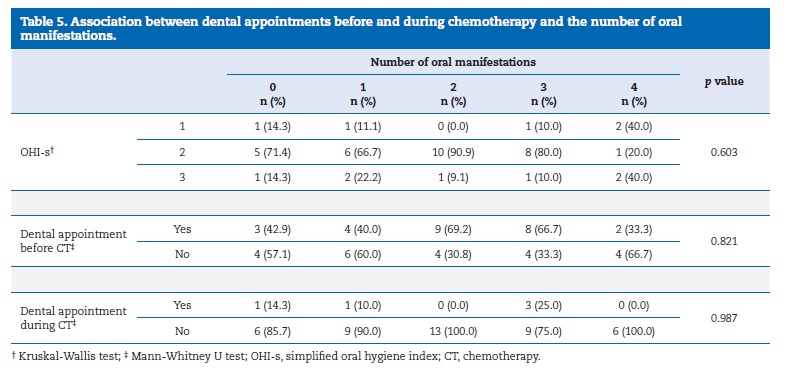
The number of oral manifestations was not associated with the presence of dental debris and calculus (p=0.603) nor with the use of dental services before CT (p= 0.821) or during CT (p=0.987) (Table 5). There was also a negative correlation, although not statistically significant (p=0.25), between the number of oral manifestations and the oral hygiene instructions received.
Discussion
This observational study was conducted in an adult population undergoing CT, to investigate the oral manifestations and symptoms during CT and whether oral health routines influence the patient’s experience of oral manifestations and symptoms and their quality of life.
Regarding the first aim of the study, many oral manifestations and symptoms were found in onco-hematology patients during CT. One study5 had reported that, after one week of CT treatment, oral symptoms increased, with mucositis, pain, dysphagia, mouth and lip dryness, dysgeusia, gingival bleeding and the ability to talk being frequently reported. The same oral manifestations were found in the present study as well as in others.18, 19 Oral ulcers were reported by one-third of the study participants but observed in one-quarter. This prevalence of oral ulcers is much higher than that of patients not doing CT.20
In a previous study, this condition increased by 22% during the CT cycle.5 Gingival inflammation and red/white lesions were also more frequent in the onco-hematology patients of this study than healthy subjects.20 Mucositis was observed in about one-fifth of the sample, while other studies had reported higher and lower frequencies of mucositis.7, 19 Difficulty in feeding was less observed in the present study as compared to others.5
Several CT-related side effects are dependent on the phase of the treatment. In the consolidating phase, where the therapy is intensified, the most common complications are mucositis, dysgeusia, xerostomia, bleeding, oral pain, opportunistic infections, neurotoxicity, and temporomandibular dysfunction, which usually occur with high prevalence and severe forms.8 One of the reasons why a low number of oral manifestations were observed in this study is probably the high number of patients that were in the maintenance phase, where a total recovery of the cells can happen.18The type of the tumor, the dose of the drugs, the number of cycles, the duration of CT, the patients’ age and their level of oral hygiene before and after therapy are factors that determine the severity of oral complications.7 It would be important to collect data related to CT protocols and tumor type but, unfortunately, these data were not available to the researchers.
Regarding the DMFT index, the results of patients doing CT were similar to those reported in healthy adults, although the decayed element was lower.20 However, the DMFT in this study was higher than the post-CT DMFT in a previous study.14 Inversely, the debris index was lower than that of the same study.14
Most patients only received recommendations for a soft diet. These patients did not receive any advice about cold diet, non-spicy diet, acid diet or avoiding tobacco and alcohol. Diet recommendations are essential to prevent exacerbation or emergence of oral symptoms. Tobacco and some drinks and foods have to be avoided, such as alcohol, coffee, tea and spicy food, and a non-cariogenic diet should be followed.21, 22
Considering all the oral health problems, oral changes can induce a decrease in quality of life. Xerostomia, which can contribute to speech difficulty, causing oral discomfort and mouth soreness and affecting the quality of life, was referred by almost all of the patients.21 A previous study had reported a much lower prevalence of xerostomia.7 Dysgeusia, which was indicated by almost half of the sample, was an expected finding due to the likely direct effect of CT in the taste buds.
Unfortunately, this change also affects the quality of life negatively, as it leads to loss of appetite and affects nutrition.21, 23
However, patients from this study reported difficulty in feeding less frequently than a healthy population.20
Several studies have reported that CT affects oral health-related quality of life.5, 22 In the present study, three-quarters of the sample reported that oral symptoms influenced their life somehow. This prevalence was higher than in another study where one-fifth of patients was affected.5 In this study, we also found that patients who had their quality of life more affected had a higher number of oral manifestations.
The duration of the CT treatment was positively correlated with the number of oral manifestations. The same was observed in other studies where the dose of the chemotherapeutic drugs and the frequency of administration were correlated with the dimension of toxicity on normal tissues.23
Regarding oral hygiene habits, almost all patients brushed their teeth at least twice a day, which is better than reports by people without cancer and oncological patients before starting CT. 20, 24 Interdental means of plaque removal were used by a quarter of the sample, with dental floss being the most used, and this prevalence is also higher compared to healthy people.20 According to some authors,22, 23, 25, 26 patients doing CT must follow an oral care plan, including the utilization of dental services. More than half of the study sample visited an oral health professional before starting CT. This visit would allow for proper counseling, treatment of existing problems and provision of appropriate preventive measures.19
Some authors5 emphasize that all patients should be seen by a dental professional before starting CT treatment. Fewer oral manifestations are found in patients with better oral health conditions and satisfactory oral hygiene.2 This idea is reinforced by other authors that believe in the importance of oral health for the prevention and reduction of oral complications of cancer treatment.3 Dental interventions should be done before starting CT to improve the quality of life.8 Because it is known that a systemic infection can lead to septic episodes in immunocompromised patients, it is essential to evaluate oral health before CT to reduce infectious outbreaks, such as removal of carious lesions and extensive restorations, treatment of periodontal disease and even tooth extractions in cases of teeth that require prolonged treatment.2 However, in the current study, it was not possible to see if oral health routines influenced the patients’ experience of oral manifestations and symptoms and their quality of life, maybe because of the small sample.
There were several limitations to this study. First of all, the sample size was small, precluding the generalization of results.
Nevertheless, this was a preliminary study that allowed some valid conclusions and insights for future studies. Another limitation was the patients’ perception of oral diseases. Patients perceive oral complications as inevitable consequences of the cancer treatment, so they do not report them nor do something to change or prevent them.27 Moreover, this study relies on self-reported data, which can be another limitation.28
It is equally important to note that we had some problems with comparing directly some results found in this study with those of other studies because studies about oral manifestations in onco-hematology adults are rare and use different methodologies.29
When the study took place, all the patients were in different phases of CT, with a different number of cycles, different durations of treatment and different types of treatment protocols, and all these can influence oral manifestations frequency. Future studies should take into account these aspects, given that different protocols induce different oral complications.
Conclusions
Based on this investigation, it is possible to infer that CT, one of the most used therapies by cancer patients, affects the oral cavity of these populations.
Many oral manifestations, such as xerostomia, dysgeusia, dental hypersensitivity, mouth ulcers and loss of appetite were noticed. Gingival changes, inflammation and hemorrhage were also present in these patients.
CT patients with a higher number of oral manifestations have their quality of life more affected. The integration of an oral health professional into the oncology team could contribute to preventing many oral manifestations through observation of the oral cavity and treatment of those diseases and symptoms before starting the CT treatment. This integration could help CT patients achieve a better quality of life and comfort before, during and after oncology therapy.
References
1. Palmela P. Salvado F. Guidelines para cuidados de saude oral em doentes oncologicos. Lisboa: Circulo Medico; 2010.
2. Velten DB, Zandonade E, Monteiro de Barros Miotto MH. Prevalence of oral manifestations in children and adolescents with cancer submitted to chemotherapy. BMC Oral Health. 2016;16:107.
3. Chen CF, Wang RH, Cheng SN, Chang YC. Assessment of Chemotherapy-Induced oral complications in children with cancer. J Pediatr Oncol Nurs. 2004;21:33-9.
4. Pereira CMMS. Quimioterapia – Manifestacoes orais e importancia do medico dentista na prevencao e tratamento [dissertation]. Lisboa: Faculdade de Medicina Dentaria da Universidade de Lisboa; 2009.
5. Ohrn K. Oral health and experience of oral care among cancer patients during radio- or chemotherapy. [Thesis]. Uppsala: Faculty of Medicine; 2001.
6. Andrade FA, Santos PSS, Freitas RR. Manifestacoes bucais em paciente com leucemia mieloide aguda (LMA). Arq Med Hosp Fac Cienc Med Santa Casa Sao Paulo. 2008;53:85-7.
7. Hespanhol FL, Tinoco EMB. Teixeira HGC. Falabella MEV. Assis NMSP. Manifestacoes bucais em pacientes submetidos a quimioterapia. Ciencia & Saude Coletiva. 2010;15(Supl:1):1085-94.
8. Zimmermann C, Meurer MI, Grando LJ, Moral JAGD. Rath IBS. Tavares SS. Dental treatment in patients with leukemia. J Oncol. 2015:571739.
9. Lionel D, Christophe L, Marc A, Jean-Luc C. Oral mucositis induced by anticancer treatments: physiopathology and treatments. Ther Clin Risk Manag. 2006;2:159-68.
10. Chemocare [Internet]. Cleveland: What is Chemotherapy. [cited 2019 Feb 14]. How long is chemotherapy given? Available from: https://www.chemocare.com/chemotherapy/what-ischemotherapy/how-long-is-chemotherapy-given.aspx
11. Cancer.org [Internet]. Atlanta: American Cancer Society. [cited 2019 Feb 14] Evolution of cancer treatments: Chemotherapy. Available from: https://www.cancer.org/cancer/cancer-basics/history-of-cancer/cancer-treatmentchemo.html
12. Morais EF, Lira JAS, Macedo RAP, Santos KS, Elias CTV, Arruda-Morais MLS. Oral manifestations resulting from chemotherapy in children with acute lymphoblastic leukemia. Braz J Otorhinolaryngol. 2014;80:78-85.
13. Murshid EZ, Azizalrahman TA, AlJohar AJ. Oral mucositis in leukemic Saudi children following chemotherapy. Saudi J Dent Res. 2017;8:79-85.
14. Hong CHL, Napenas JJ, Hodgson BD, Stokman MA, Mathers-Stauffer V, Elting LS, et al. A systematic review of dental disease in patients undergoing cancer therapy. Support Care Cancer. 2010;18:1007-21.
15. Silveira A, Goncalves J, Sequeira T, Ribeiro C, Lopes C, Monteiro E, Pimentel FL. Avaliacao da Qualidade de Vida em Doentes com Patologia Oncologica da Cabeca e do Pescoco. Modelo de validacao da versao Electronica Portuguesa do EORTC-QLC C30 e EORTC – H&N35. Acta Med Port. 2011;24(S2):347-54.
16. World Health Organization. WHO Oral Health Assessment Form for Adults. In: WHO Editors. Oral health surveys: basic methods. 5th ed. Geneva: WHO Press; 2013:83-84.
17. Greene JC, Vermillion, JR. The simplified oral hygiene index. J Am Dent Assoc. 1964;68:7-13.
18. Lopez BC, Esteve CG, Perez MGS. Dental treatment considerations in the chemotherapy patient. J Clin Exp Dent. 2011;3:e31-42.
19. Goyri BLM, Ramos MEC, Perez EE. Estomatotoxicidad bucal inducida por quimioterapia. Rev Odontol Mex. 2014,18:89-95.
20. Direcao Geral da Saude [Internet]. Lisboa: DGS [cited 2018 Dec 27]. III Estudo Nacional de Prevalencia das Doencas Orais 6, 12, 18, 35-44 e 65-74 anos. Relatorio Apresentacao de dados; 2015. Available from: https://www.dgs.pt/documentose-publicacoes/iii-estudo-nacional-de-prevalencia-dasdoencas-orais.aspx
21. Moreira ASS. Complicacoes orais da radioterapia e quimioterapia – implicacoes na qualidade de vida [dissertation]. Porto: Faculdade de Medicina Dentaria da Universidade do Porto; 2016.
22. Persson L. Daily life problems from a nursing perspective in patients with acute leukaemia or highly malignant lymphoma [dissertation]. Lund: Centre of Caring Sciences – The Medical Faculty Lund University; 1998.
23. Poulopoulos A, Papadopoulos P, Andreadis D. Chemotherapy: oral side effects and dental interventions – a review of the literature. Stomatological Dis Sci. 2017; 1:35-49.
24. National Institute of Dental and Craniofacial Research [Internet]. Bethesda (MD): Oral Health, Cancer Care and You. [cited 2019 Feb14]. Oral complications of cancer treatment: What the Dental Team can Do;2009. Available from: https://edentalsites.com/roswellperiodontics/files/2015/06/Oncology-Complications-of-Cancer-Treatment.pdf
25. Galindo MPL, Bagan JV, Soriano YJ, Alpiste F, Camps C. Clinical evaluation of dental and periodontal status in a group of oncological patients before chemotherapy. Med Oral Patol Oral Cir Bucal. 2006;11:E17-21.
26. Kroetz FM, Czlusniak GD. Alteracoes bucais e condutas terapeuticas em pacientes infanto-juvenis submetidos a tratamentos anti-neoplasicos. Publication UEPG Ciencias Biologicas e da Saude. 2003;9:41-8.
27. Pereira LJ, Caputo JB, Castelo PM, Andrade EF, Marques LS, de Paiva SM, et al. Oral physiology and quality of life in cancer patients. Nutr Hosp. 2015;31:2161-6.
28. Bhandari A, Wagner T. Self-reported utilization of health care services: improving measurement and accuracy. Med Care Res Ver. 2006;63:217-35.
29. Cleeland CS, Mendoza TR, Wang XS, Chou C, Harle MT, Morrissey M, Engstrom MC. Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer. 2000;89:1634-46.
Jessica Gilberto Santilal
E-mail address: jessica_gilberto@hotmail.com
Ethical disclosures
Protection of human and animal subjects. The authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of data. The authors declare that they have followed the protocols of their work center on access to patient data and for its publication.
Right to privacy and informed consent. The authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflict of interest
The authors have no conflicts of interest to declare.
Article history:
Received 17 April 2019
Accepted 12 October 2019
Available online 29 October 2019
