
Revista Portuguesa de Estomatologia, Medicina Dentária e Cirurgia Maxilofacial
SPEMD | 2019 | 60 (3) | 111-117
Original research
Cytotoxic effects of hydrogen peroxide on periodontal cells
Efeitos citotóxicos do peróxido de hidrogénio em células periodontais
a Faculdade de Medicina Dentária, Universidade de Lisboa, Lisboa, Portugal
b GIBBO – Grupo de Investigação em Biologia e Bioquímica Oral – Faculdade de Medicina Dentária, Universidade de Lisboa, Lisboa, Portugal
c LIBPhys-FCT UID/FIS/04559/2013
Joana Marques - joanafariamarques@gmail.comjoanafariamarques@gmail.com
Article Info
Rev Port Estomatol Med Dent Cir Maxilofac
Volume - 60
Issue - 3
Original research
Pages - 111-117
Go to Volume
Article History
Received on 20/04/2019
Accepted on 10/09/2019
Available Online on 30/09/2019
Keywords
Original research
Cytotoxic effects of hydrogen peroxide on periodontal cells
Efeitos citotoxicos do peroxido de hidrogenio em celulas periodontais
Andreia Vieiraa,b, Joana Marquesa,b,*, Mariana Cruza,b, Carlota Mendonçaa,b, Duarte Marquesa,b,c, António Mataa,b,c
a Faculdade de Medicina Dentária, Universidade de Lisboa, Lisboa, Portugal
b GIBBO – Grupo de Investigação em Biologia e Bioquímica Oral – Faculdade de Medicina Dentária, Universidade de Lisboa, Lisboa, Portugal
c LIBPhys-FCT UID/FIS/04559/2013
http://doi.org/10.24873/j.rpemd.2019.09.455
Abstrat
Objectives: To evaluate the in vitro cytotoxic effects of hydrogen peroxide (H2O2) on periodontal cells, based on the cellular viability and morphology of immortalized osteoblast and gingival fibroblast cultures, with different exposure times and concentrations.
Methods: Immortalized human gingival fibroblast and human fetal osteoblast cell lines were cultured, separately, in 96-well plates. After reaching confluency, they were exposed to H2O2 solutions at 16 different concentrations ranging between 0.05 μg/ml and 10 μg/ml for 1 h, 24 h or 72 h in triplicate assays (n=24), using culture media alone as the control. Cell viability was measured by previously established fluorometric methods, using a resazurin-based assay, and cell morphology by using an inverted microscope with integrated phase-contrast optics. Data were statistically analyzed using a one-way ANOVA with Tukey’s and Dunnet’s post hoc tests and Pearson correlation coefficient (r), as appropriate (α=0.05).
Results: H2O2 induced a decrease in cell viability to below 50% in fibroblasts and around 50% in osteoblasts, in all tested concentrations after 1h exposure, and a decrease in cell viability to above 70% after 24 h and 72 h (P<0.05). A significant negative correlation was detected between H2O2 and cell viability at 1 h and 72 h for osteoblast (r=-0.471) and HGF (r=-0.12) cells, respectively. The cell morphology analysis showed cell detachment and lower cell density, in agreement with these findings.
Conclusions: H2O2 induced cell alterations with moderate to severe cytotoxic effects in osteoblast and gingival fibroblasts.
Keywords: Cytotoxicity, Fibroblast, Gingiva, Hydrogen peroxide, Osteoblast
Resumo
Objectivos: Avaliar in vitro a citotoxicidade do Peroxido de Hidrogénio (H2O2) em células periodontais através do efeito de soluções H2O2 na viabilidade e morfologia de culturas de fibroblastos gengivais e osteoblastos humanos imortalizados, em diferentes concentrações e tempos de exposição.
Métodos: Foram usadas linhagens imortalizadas de fibroblastos gengivais e osteoblastos fetais humanos, as quais foram cultivadas, separadamente, em placas de 96 pocos, que ao atingir a confluência, foram expostas a concentrações de H2O2 de 0,05 μg/ml a 10 μg/ml (16 concentrações diferentes), durante 1 h, 24 h ou 72 h, em ensaios triplicados (n=24), sendo utilizado exclusivamente meio de cultura como controlo. A viabilidade celular foi avaliada por métodos fluorometricos previamente descritos através da conversão da resazurina e a morfologia celular por microscopia otica invertida com contraste de fase. Os dados foram analisados estatisticamente através do teste ANOVA one-way com post-hoc de Tukey e Dunnet’s e do coeficiente de correlação de Pearson (r) conforme apropriado (α=0,05).
Resultados: O H2O2 induziu uma reducao da viabilidade, superior a 50% tanto nos fibroblastos como nos osteoblastos, visivel em todas as concentrações testadas após 1h de exposição e superior a 70% a 24h e 72h (p<0,05). Foi detetada uma correlação significativa e negativa aos tempos 1h e 72h para osteoblastos (r=-0,471) e fibroblastos (r=-0,12), respetivamente. A analise das micrografias obtidas apresentou destacamento celular e menor densidade celular em concordancia com estes resultados.
Conclusão: A exposição ao H2O2 resultou em alterações celulares e efeitos citotoxicos de moderados a severos em fibroblastos gengivais e osteoblastos.
Palavras-chave: Citotoxicidade, Fibroblasto, Periodonto, Peroxido de hidrogénio, Osteoblasto
Introduction
Hydrogen peroxide (H2O2) is a well-described reactive oxygen species (ROS) commonly used in Dentistry procedures.1 Namely, it is used in periodontal and peri-implantitis treatments including implant surface decontamination, as well as in tooth whitening products, mouth rinses and toothpastes.2, 6 H2O2 concentrations depend on the type of treatment: 30 μg/ml for peri-implantitis treatments and 30 μg/ml to 380 μg/ml for tooth bleaching. However, it has been well-described that H2O2 has a broad spectrum of biological effects that raise several concerns regarding its safety, even at low concentrations. Among those biological effects is the overproduction of ROS, which results in DNA, lipid and protein oxidation that may eventually lead to cell necrosis or apoptosis mechanisms in oral cells.7, 8 During oral care procedures, periodontal cells are potentially exposed to H2O2 from endogenous and exogenous sources.
In periodontitis and peri-implantitis treatments, H2O2 is conventionally used as a decontaminating agent. A validated protocol for peri-implantitis is based on a 30-μg/ml solution of H2O2 applied on the implant surface.3, 9, 10 During these treatments, connective tissue and bone in the peri-implant space are in contact with H2O2, thus raising questions on its toxicity and impact on tissue healing.
H2O2 is also the main active product in bleaching treatments. It has been demonstrated in vitro that, during internal whitening procedures, H2O2 is able to diffuse from the pulp chamber to the root surface.(11, 12) This observation is supported by the documented risk of external root resorption associated with non-vital whitening procedures, in which the diffusion of H2O2 is considered to be the most probable etiological factor.13, 14 While the potential toxicity of H2O2 in external bleaching techniques has been previously assessed in pulp cells, little is known on the risks presented to periodontal cells by external and internal bleaching procedures, which may release up to 0.34 to 4.4 μg/ml of H2O2 into the periodontal space after 24 h of exposure.1, 15, 16
These considerations suggest a potential risk to periodontal cells, especially fibroblasts and osteoblasts. Human gingival fibroblasts (HGF) play an important role in the homeostasis of periodontal tissues since fibroblasts have an intrinsic ability to differentiate into other cells.17 Osteoblasts, on the other hand, are the primary cells responsible for the formation of bone, by synthesizing the components of the extracellular matrix and regulating its mineralization.18 While the current literature has focused mostly on the effects of H2O2 on the dental pulp after bleaching procedures, the effects of H2O2 on HGFs and osteoblasts may also be assessed using odontoblasts and dental pulp cells as models.
Previous studies using HGFs have shown that a high concentration of H2O2 (15% v/v) can delay cell division and induce alterations in cell morphology.19 On the other hand, in studies using osteoblasts, H2O2 has been described as inhibiting osteoblastic differentiation and inducing bone loss.20 Previous reports have focused on high H2O2 concentrations, but the response of periodontal tissue cells to low-dose H2O2 (lower than 10 μg/ml), as observed in clinical conditions (peri-implantitis treatment, diffusion during whitening procedures), has not been described.
The aim of this study was to evaluate the cytotoxicity of H2O2 solutions with different concentrations on periodontal cells, using fibroblast and osteoblast cell lines. The secondary aims were to assess the effect of the H2O2 concentration on cell viability and cell morphology in different exposure times. The null hypotheses to be tested were: 1) Exposure to H2O2 does not decrease osteoblast viability; 2) Exposure to H2O2 does not decrease gingival fibroblast viability; 3) H2O2 concentration is not correlated with osteoblast viability; 4) H2O2 concentration is not correlated with gingival fibroblast viability.
Material and methods
HGF from an hTERT-immortalized cell line (ABMR Canada) were cultured at 37°C in a 100% humidified atmosphere containing 5% CO2. The cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM; LonzaR, Basel, Switzerland) supplemented with 10% fetal bovine serum (FBS; BiowestR, Nuaille, France) and 1% penicillin/streptomycin (BiowestR, Nuaille, France). At 80% confluence, the cells were trypsinated, centrifuged at 800 rpm and resuspended in culture media. Passage 7 was used for all the tests as displaying typical cell behavior for this cell line. All experiments were conducted at 37°C.
Human fetal osteoblasts hFOB 1.19 and a SV40-immortalized cell line (ATCC; American Culture Collection, Manassas, VA, USA) were used. Cells were cultured at 35°C in an atmosphere of 5% CO2 and 100% humidity, in a culture medium composed of a 1:1 mixture of Ham’s F12 Medium (Sigma-Aldrich 51651C, Hampshire, UK.) and DMEM (Biowhittaker, Lonza, Walksville, USA) supplemented with 0.3 mg/ml geneticin – G418 (Roche, Indiana, USA) and 10% FBS (Biowest, Nuaille, France) until reaching 80% confluence. Cells were then trypsinated, centrifuged at 800 rpm and resuspended in culture media at an adequate density for the assays. Passage 8 was used for all tests, as displaying typical cell behavior for this cell line. All the experiments were conducted at 37oC, which is the restrictive temperature for hFOB 1.19, since primary cell behavior is exhibited at this temperature, with little cell division and increased differentiation, thus providing a more representative model.
Both cells were cultured, separately, in 96-well plates with a cell density of 5.0 x 103 cells/well. After 24 h of incubation, the culture medium was replaced by an H2O2 solution for 1 h, 24 h or 72 h.
From the initial 30 μg/ml H2O2 solution (Sigma-Aldrich, St. Louis, MO, USA), 16 H2O2 solutions were prepared with concentrations ranging between 0.05 μg/ml and 10 μg/ml (0.05 μg/ml; 0.10 μg/ml; 0.15 μg/ml; 0.20 μg/ml; 0.25 μg/ml; 0.30 μg/ml; 0.35 μg/ml; 0.40 μg/ml; 0.50 μg/ml; 0.75 μg/ml; 1.0 μg/ml; 1.5 μg/ml; 3.0 μg/ml; 5.0 μg/ml; 10.0 μg/ml). These concentrations were determined according to the potential H2O2 range of exposure during clinical treatment and, thus, IC50 assessment was not performed. The solutions were diluted with an appropriate medium according to the cell line. Cells were incubated with H2O2 solutions for 1 h, 24 h and 72 h, and, in order to simulate clinical conditions, solutions were not renovated, and culture media alone was used as control. Each group had a final sample size of 24 wells, based on triplicate assays of n=8 wells each.
Cytotoxicity was assessed based on cell viability and proliferation assays using a 20% resazurin solution (Sigma-Aldrich, St. Louis, MO, USA), according to the ability of living cells to irreversibly convert a redox dye (resazurin) into a fluorescent final product (resorufin). The conversion rate was measured as the fluorescence intensity after 1 h, 24 h and 72 h of culture. After incubation at 37°C for 3 h, a cell-viability assay was performed following the manufacturer’s instructions, and the fluorescence intensity was recorded at excitation/emission wavelengths of 560/590 nm in a luminescence spectrometer (PerkinElmer LS 50B, Waltham MA, USA). The results were expressed in arbitrary units (A.U.). The mean of three consecutive measurements in each well was considered as the final result. Cytotoxicity was evaluated based on the cell viability relative to the controls as a percentage, according to the following formulas:

For cell morphology evaluation, the cells were observed under an inverted microscope with integrated phase-contrast optics (Olympus CK2, Tokyo, Japan). The micrographs were obtained at 250x magnification with a NIKONR D60 (Tokyo, Japan) camera mounted with an appropriate lens adapter, and were analyzed by two independent observers, considering cell density, adhesion and morphology.
Statistical analysis was performed using IBMR SPSSR Statistics 24, Inc., Chicago, IL, EUA. Normality was assessed using the Kolmogorov-Smirnov test. The differences of viability between the H2O2 concentrations at different time points were analyzed by one-way analysis of variance (ANOVA), using Tukey’s and Dunnet’s post hoc tests, as appropriate. Pearson correlation coefficients were used to correlate H2O2 concentrations, exposure time and cell viability, with P<0.05 considered as significant. The strength of the resulting correlations was described using established criteria.21 The results were expressed as the mean ± 95% confidence interval. The significance was set at an alpha value of 0.05 and a beta value of 0.80.
Results
A decrease in cell viability was observed for all H2O2 concentrations in both cell lines. In HGF, it was more marked in the 0.05-0.75 μg/ml range at 1 h, 24 h and 72 h (P<0.05), while in osteoblasts it was less pronounced in the lowest H2O2 concentration (0.05 μg/ml) with a 23.5% (14.49%; 32.51%) decrease.
The lowest viability values occurred at a concentration of 0.35 μg/ml and remained stable up to the highest concentration (10.0 μg/ml).
After 1 h of exposure to H2O2, viability decreased to below 50% in HGF (Figure 1A) and around 50% in osteoblasts (Figure 2A). After 24 h of incubation (Figure 1B and 2B), an increase in the number of cells in the control wells was evident. However, in the first tested concentration (0.05 μg/ml), viability significantly decreased to approximately 25% in HGFs (P<0.05) and osteoblasts (P<0.05). After 72 h of H2O2 exposure (Figure 1C and 2C), the differences detected between the control and the concentrations studied reflected a decrease of over 80% viability in HGFs and osteoblasts (P<0.05) without statistically significant differences between the different concentrations tested.
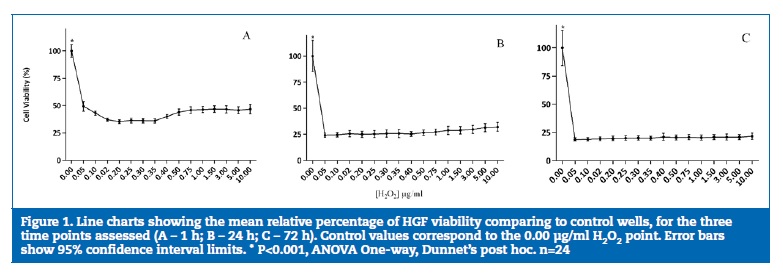
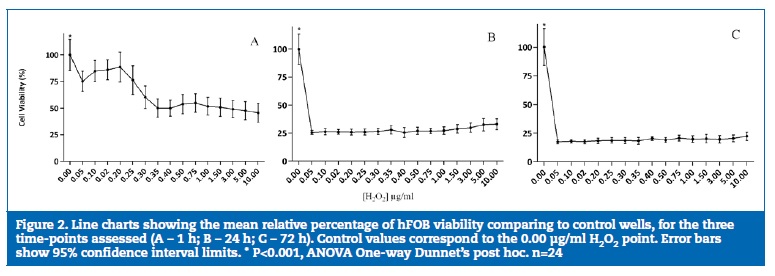
A very small but significant negative correlation (r =-0.164, P<0.01) between H2O2 concentration and cell viability was reported for osteoblasts. When separate time points were considered, H2O2 concentration was significantly correlated with osteoblast viability at 1h of exposure (r=-0.471, P<0.01) and with HGF viability at 72h of exposure (r = -0.12, P<0.05). When cell viability was correlated to exposure time, a significant negative correlation (P<0.01) with moderate effect was detected for both cell lines, with r =-0.573 for osteoblasts and r=-0.403 for HGF, corresponding to lower viability with exposure time increase.
HGF micrographs showed that, as a result of the exposure to increasing H2O2 concentrations, cell morphology changes were observed from fusiform to rounded and flattened cells, with various stages of cell detachment in all the exposure times (1 h, 24 h and 72 h). Figures 3 and 4 show the micrographs obtained using phase-contrast microscopy for the osteoblasts and HGF cultures, respectively. In the control wells, normal osteoblast and fibroblast morphologies were observed, showing adhered elongated cells spread with filamentous extensions, which indicate a correct cell attachment.
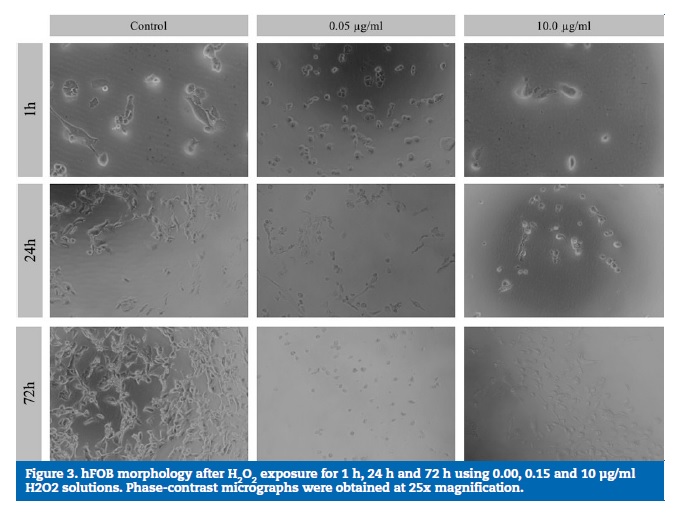
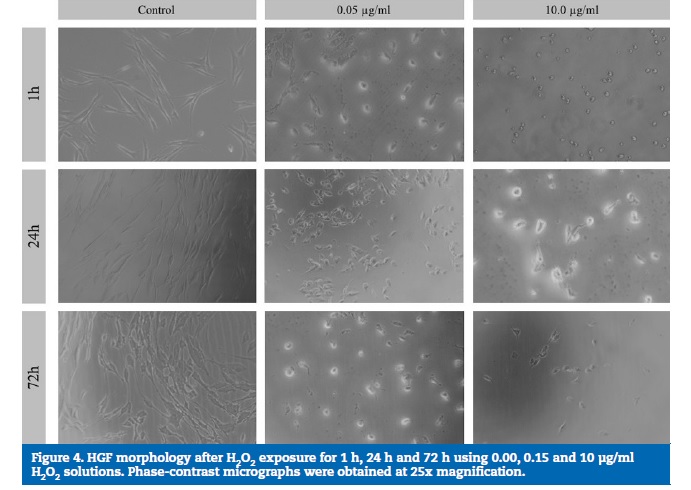
However, with higher H2O2 concentrations, alterations in cell morphology were evident, with round and flattened cells, which are compatible with various stages of cell detachment.
A perceived increase in the cell density in the control wells, but not in the H2O2-exposed wells, was observed from 1 h to 72 h.
Discussion
H2O2 has been widely used for a number of preventive and therapeutic applications in Dentistry.1, 6 While generally regarded as a safe agent for these applications, H2O2 has a strong oxidant potential. Its effects in teeth and pulpal tissues have been well studied;22, 27 however, an exhaustive screening of the potential toxicity of H2O2 within the clinically relevant concentration range in periodontal cells has not yet been performed. In the present work, we evaluated the in vitro effects of H2O2 exposure on the cell viability of osteoblasts and fibroblasts. Cytotoxicity was classified, in accordance with the ISO standard 10993-5:2009, as non-cytotoxic at > 80% cell viability; slightly cytotoxic at 80-50% cell viability; moderately cytotoxic at 50-30% cell viability; and severely cytotoxic at <30% cell viability (ISO 10993-5:2009).
The results of our study showed, for the first time, that, in both the osteoblast and gingival fibroblast cell lines tested, exposure to a 0.05-μg/ml solution of H2O2 (10-fold lower than the reportedly safe concentration of 0.68 μg/ml) elicited a cell-viability decrease of up to 50% (P<0.05), thus rejecting the first two null hypotheses. This effect was more pronounced with longer incubation times. According to some authors, exposure to H2O2 concentrations lower than 0.68 μg/ml is considered safe regardless of the cell type, resulting in limited effects in many animal cells.28 A significant impact on the cell viability would only be expected in exposures to concentrations over 1.7 μg/ml.7 As stated before, our results contradict this assumption, suggesting that H2O2 toxicity for periodontal cells might be underestimated. This divergence could be explained by the use of distinct and more-resistant cell lines in these previous studies, or by other differences in experimental design, including broader concentration ranges or shorter exposure times.
Different toxicity effects were observed in the two cell types used in this assay. After the exposure of HGF to the lowest-concentration H2O2 solution (0.05 μg/ml), mean viability decreases of 50% – slightly cytotoxic – (1 h) and 80% – severely cytotoxic (24 and 72 h) – were observed. In previous studies with human fibroblast cell lines, concentrations of H2O2 ranging between 1.7 and 42.5 μg/ml resulted in a proliferation decrease, morphology alterations and cell death.(29 - 34)
However, these studies were based on shorter exposure times (90 seconds), while, in our study, the shortest exposure was 1 h, and most were performed in types of fibroblasts other that gingival fibroblasts. Our results presented a similar decrease in cell viability at a 100-fold lower concentration of H2O2 for the HGF cell line (0.05 μg/ml), thus suggesting that the tolerance threshold for gingival fibroblasts may be lower than previously reported, considering exposure times comprised between 1 h and 72 h.1, 19 However, this effect might be specifically related to the cell line used in this study, which may be more sensitive to H2O2 exposure than other cell lines and primary cells.
Higher resistance to H2O2 after 1 h of exposure, with non-cytotoxic to slightly cytotoxic effects (25-50% viability decrease) but similar severely cytotoxic effects in longer exposures, was observed in hFOBs. A previous study with hFOB cells reported a safe threshold of a 24-h exposure to 3.4 μg/ml H2O2.35 Our results suggest that this limit is potentially below a concentration of 0.05 μg/ml of H2O2 in osteoblasts, particularly after 24 h of exposure. This difference may result from the use of a different method for viability assessment, as the authors of the previous study used formazan reduction.
While these methods are generally considered equivalent, some reports state different performances of resazurin and formazan methods, with resazurin generally causing higher sensitivity.36, 37
A small negative overall correlation between H2O2 concentration and cell viability was detected in osteoblasts, specifically at 1 h of exposure (moderate effect). It can be therefore speculated that osteoblasts may have a dose-dependent response to short-term H2O2 exposures. The results also suggest that, within this range of concentrations, there is a dose-dependent response in the viability of HGFs at 72 h of exposure (small effect), thus rejecting the third and fourth null hypotheses.
An important limitation of our study is that only one cell line was used for each cell type. Thus, further studies using different cell lines and primary osteoblasts or fibroblasts should be undertaken to confirm these findings. IC50 was not calculated because the scope of this study was to evaluate the effects of clinically relevant concentrations on cell viability.
However, further studies should characterize the toxicological profile of H2O2 exposure in these cell types in order to fully elucidate on the safe threshold concentration of this agent, as well as its impacts on cell function and differentiation. Finally, this was an in vitro study, with the inherent limitations that turn direct extrapolation to in vivo impossible, namely the absence of antioxidant mechanisms, or the rate of H2O2 degradation. Within the limits of this study, the results suggest that H2O2 exposure in the range of concentrations used in peri-implantitis debridement and in internal bleaching procedures, potentially reaching the periodontal space, may severely decrease periodontal cell viability. These findings raise new questions regarding H2O2 safety for those applications. Whether this cytotoxicity happens in vivo and to what extent H2O2 exposure impacts cell function is yet to determine. These observations need to be integrated into more complex models to clarify the role of endogenous and exogenous antioxidant systems in reverting these alterations.
Conclusions
The results of this study suggest that direct exposure to H2O2 within clinically relevant concentrations resulted in moderate to severe cytotoxic effects in periodontal cells – osteoblasts and gingival fibroblasts in vitro. A small negative correlation between H2O2 concentration and cell viability was observed in osteoblasts but not in fibroblasts.
Further studies are necessary to determine the exact safety threshold of the direct application of H2O2 on these cells, as well as its impact on cell differentiation and function.
References
1. Walsh LJ. Safety issues relating to the use of hydrogen peroxide in dentistry. Aust Dent J. 2000;45:257-69.
2. Jepsen K, Jepsen S, Laine ML, Anssari Moin D, Pilloni A, Zeza B, et al. Reconstruction of Peri-implant Osseous Defects: A Multicenter Randomized Trial. J Dent Res. 2015;95:58-66.
3. Wheelis SE, Gindri IM, Valderrama P, Wilson TG, Huang J, Rodrigues DC. Effects of decontamination solutions on the surface of titanium: investigation of surface morphology, composition, and roughness. Clin Oral Implants Res. 2016;27:329-40.
4. Dahl JE, Pallesen U. Tooth Bleaching–a Critical Review of the Biological Aspects. Crit Rev Oral Biol Med. 2003;14:292-304.
5. Li Y. Safety controversies in tooth bleaching. Dent Clin North Am. 2011;55:255-63.
6. Carey CM. Tooth whitening: What we now know. J Evid Based Dent Pract. 2014;14(Suppl.):70-6.
7. Halliwell B, Clement MV, Ramalingam J, Long LH. Hydrogen Peroxide. Ubiquitous in Cell Culture and In vivo? IUBMB Life. 2000;50:251-7.
8. Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44-84.
9. Henderson E, Schneider S, Petersen FC, Haugen HJ, Wohlfahrt JC, Ekstrand K, et al. Chemical debridement of contaminated titanium surfaces: An in vitro study. Acta Odontol Scand. 2013;71:957-64.
10. Gosau M, Hahnel S, Schwarz F, Gerlach T, Reichert TE, Burgers R. Effect of six different peri-implantitis disinfection methods on in vivo human oral biofilm. Clin Oral Implants Res. 2010;21:866-72.
11. Lee GP, Lee MY, Lum SO, Poh RS, Lim KC. Extraradicular diffusion of hydrogen peroxide and pH changes associated with intracoronal bleaching of discoloured teeth using different bleaching agents. Int Endod J. 2004;37:500-6.
12. Palo R, Bonetti-Filho I, Valera M, Camargo C, Camargo S, Moura-Netto C, et al. Quantification of Peroxide Ion Passage in Dentin, Enamel, and Cementum After Internal Bleaching With Hydrogen Peroxide. Oper Dent. 2012;37:660-4.
13. Patel S, Kanagasingam S, Pitt Ford T. External Cervical Resorption: A Review. J Endod. 2009;35:616-25.
14. Naik S, Tredwin CJ, Scully C. Hydrogen peroxide toothwhitening (bleaching): Review of safety in relation to possible carcinogenesis. Oral Oncol. 2006;42:668-74.
15. Goldberg M, Grootveld M, Lynch E. Undesirable and adverse effects of tooth-whitening products: A review. Clin Oral Investig. 2010;14:1-10.
16. Madhu KS, Hegde S, Mathew S, Lata DA, Bhandi SH, Shruthi N. Comparison of Radicular Peroxide Leakage from four Commonly used Bleaching agents following Intracoronal Bleaching in Endodontically treated teeth – An In Vitro Study. J. Int Health. 2013;5:49-55.
17. Choe Y, Yu JY, Son YO, Park SM, Kim JG, Shi X et al. Continuously Generated H2O2 Stimulates the Proliferation and Osteoblastic Differentiation of Human Periodontal Ligament Fibroblasts. J Cell Biochem. 2012;113:1426-36.
18. Pacios S, Xiao W, Mattos M, Lim J, Tarapore RS, Alsadun S, et al. Osteoblast Lineage Cells Play an Essential Role in Periodontal Bone Loss Through Activation of Nuclear Factor-Kappa B. Sci Rep. 2015;5:1-12.
19. Furukawa M, K-Kaneyama JR, Yamada M, Senda A, Manabe A, Miyazaki A. Cytotoxic Effects of Hydrogen Peroxide on Human Gingival Fibroblasts In Vitro. Oper Dent. 2015;40:430-9.
20. Lee DH, Lim B, Lee Y, Yang H. Effects of hydrogen peroxide (H2O2) on alkaline phosphatase activity and matrix mineralization of odontoblast and osteoblast cell lines. Cell Biol Toxicol. 2006;22:39-46.
21. Hinkle DE, Wiersma W, Jurs S. Applied statistics for the behavioral Sciences. 5th ed. Boston, US: Houghton Mifflin; 2002.
22. Benetti F, Gomes-Filho JE, Ferreira LL, Ervolino E, Briso ALF, Sivieri-Araujo G, et al. Hydrogen peroxide induces cell proliferation and apoptosis in pulp of rats after dental bleaching in vivo: Effects of the dental bleaching in pulp. Arch Oral Biol. 2017;81:103-9.
23. Vaz M, Lopes L, Cardoso P, Souza J, Batista A, Costa N, et al. Inflammatory response of human dental pulp to at-home and in-office tooth bleaching. J Appl Oral Sci. 2016;24:509-17.
24. Cartagena AF, Parreiras SO, Loguercio AD, Reis A, Campanha NH. In-office bleaching effects on the pulp flow and tooth sensitivity – case series. Braz Oral Res. 2015;29:1-6.
25. Ortecho-Zuta U, de Oliveira Duque C, Leite M, Bordini E, Basso F, Hebling J, et al. Effects of Enzymatic Activation of Bleaching Gels on Hydrogen Peroxide Degradation Rates, Bleaching Effectiveness, and Cytotoxicity. Oper Dent. 2019; 44:414-23.
26. Borges A, Zanatta R, Barros A, Silva L, Pucci C, Torres C. Effect of Hydrogen Peroxide Concentration on Enamel Color and Microhardness. Oper Dent. 2015;40:96-101.
27. Llena C, Esteve I, Forner L. Effect of hydrogen and carbamide peroxide in bleaching, enamel morphology, and mineral composition: In vitro study. J Contemp Dent Pract. 2017;18:576-82.
28. Cochrane CG. Cellular injury by oxidants. Am J Med. 1991;91(3C):23S-30S.
29. Tipton DA, Braxton SD, Dabbous MK. Effects of a Bleaching Agent on Human Gingival Fibroblasts. J Periodontol. 1995;66:7-13.
30. Grasso S, Scifo C, Cardile V, Gulino R. Renis M. Adaptive Responses to the Stress Induced by Hyperthermia or Hydrogen Peroxide in Human Fibroblasts. Exp Biol Med. 2003;228:491-8.
31. Gutierrez-Venegas G, Arreguin-Cano JA, Arroyo-Cruz R, Villeda-Navarro M, Mendez-Mejia JA. Activation of ERK1/2 by protein kinase C-alpha in response to hydrogen peroxideinduced cell death in human gingival fibroblasts. Toxicol In Vitro. 2010;24:319-26.
32. Tipton DA, Braxton SD, Dabbous MK. Role of Saliva and Salivary Components as Modulators of Bleaching Agent Toxicity to Human Gingival Fibroblasts In Vitro. J Periodontol. 1995;66:766-74.
33. Xiang J, Wan C, Guo R, Guo D. Is Hydrogen Peroxide a Suitable Apoptosis Inducer for All Cell Types? Biomed Res Int. 2016;2016:7343965.
34. Gutierrez-Venegas G, Guadarrama-Solis A, Munoz-Seca C, Arreguin-Cano JA. Hydrogen peroxide-induced apoptosis in human gingival fibroblasts. Int J Clin Exp Pathol. 2015;8:15563-72.
35. Kandler B, Maitz P, Fischer MB, Watzek G, Gruber R. Platelets can neutralize hydrogen peroxide in an acute toxicity model with cells involved in granulation tissue formation. Bone. 2005;36:671-7.
36. Hamid R, Rotshteyn Y, Rabadi L, Parikh R, Bullock P. Comparison of alamar blue and MTT assays for high through-put screening. Toxicol In Vitro. 2004;18:703-10.
37. Langdon SP. Cancer Cell Culture. 1st ed. Totowa, NJ: Humana Press, 2004.
Joana Faria Marques
E-mail address: joanafariamarques@gmail.com
Ethical disclosures
Protection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of data. The authors declare that no patient data appear in this article.
Right to privacy and informed consent. The authors declare that no patient data appear in this article.
Conflict of interest
The authors have no conflicts of interest to declare.
Article history:
Received 20 April 2019
Accepted 10 September 2019
Available online 30 September 2019
