
Revista Portuguesa de Estomatologia, Medicina Dentária e Cirurgia Maxilofacial
SPEMD - Rev Port Estomatol Med Dent Cir Maxilofac | 2018 | 59 (4) | 181-190
Original research
Self-rated health and oral health in type 2 diabetic patients – a case-control study
Auto-avaliação de saúde e saúde oral em pacientes diabéticos tipo 2 – estudo caso-controle
a Faculty of Dentistry, University of Porto, Porto, Portugal
b Faculty of Health Sciences, University Fernando Pessoa, Porto, Portugal
c Institute of Public Health, University of Porto (ISPUP), Porto, Portugal
d Fernando Pessoa Energy, Environment and Health Research Unit (FP-ENAS), Porto, Portugal
e LAQV/REQUIMTE, University of Porto, Porto, Portugal
f Primary Healthcare Centre (USF) Barão do Corvo, ACES, Gaia, Portugal
g The Epidemiology Research Unit (EPIUnit), Porto, Portugal
Maria Conceição Manso - cmanso@ufp.edu.pt
Article Info
Rev Port Estomatol Med Dent Cir Maxilofac
Volume - 59
Issue - 4
Original research
Pages - 181-190
Go to Volume
Article History
Received on 09/06/2018
Accepted on 27/12/2018
Available Online on 06/02/2019
Keywords
Original research
Self-rated health and oral health in type 2 diabetic patients – a case-control study
Auto-avaliação de saúde e saúde oral em pacientes diabéticos tipo 2 – estudo caso-controle
José Frias-Bulhosaa,b,c, Maria Conceição Mansob,d,e,*, Carla Lopes Motaf, Paulo Meloa,c,g
a Faculty of Dentistry, University of Porto, Porto, Portugal
b Faculty of Health Sciences, University Fernando Pessoa, Porto, Portugal
c Institute of Public Health, University of Porto (ISPUP), Porto, Portugal
d Fernando Pessoa Energy, Environment and Health Research Unit (FP-ENAS), Porto, Portugal
e LAQV/REQUIMTE, University of Porto, Porto, Portugal
f Primary Healthcare Centre (USF) Barão do Corvo, ACES, Gaia, Portugal
g The Epidemiology Research Unit (EPIUnit), Porto, Portugal
http://doi.org/10.24873/j.rpemd.2018.11.423
Abstract
Objectives: To analyze whether there are differences between self-rated general health (SRH) and self-rated oral health (SROH) between individuals with type 2 diabetes mellitus (DM2) and without DM2, and to explore the general and oral conditions associated with major impacts on general health (GH) and oral health (OH) self-perception.
Methods: After obtaining ethical approval and informed consent, the sample included 343 DM2 patients (DM2 group) and 323 controls (nDM2 group), all adult volunteers. Data was obtained from clinical records and oral health examination, using WHO criteria and a questionnaire to rate their own GH and OH on a Likert 5-point scale. Inference analysis was conducted using parametric and non-parametric tests and logistic multivariable regression (MLR) (α=0.05).
Results: DM2 patients’ perception of own GH was significantly worse than nDM2’s (p<0.001). The MLR showed that the risk factors for a worse perceived GH were calculus and oral hygiene at night in nDM2, and HTA, dyslipidaemia and at least one missing tooth in DM2. Both SRH and SROH were significantly (p<0.001) often classified as “bad” or “very bad” in DM2. Calculus and pockets ≥4mm were identified as increased risk factors (OR=3.55, p=0.049; OR=4.32, p=0.025, respectively) and DMFT>0, calculus and gingival recession were associated (p<0.001) with “bad” or “very bad” SRH and SROH in the MLR.
Conclusions: Individuals with type 2 diabetes mellitus showed a worse self-perception of own general health and oral health than individuals without this pathology, and oral health was generally self-rated worse than general health.
Keywords: Case-control study,Primary health care, Self-rated health, Self-rated oral health,Type 2 diabetes.
Resumo
Objetivos: Verificar existência de diferenças entre a autoavaliação da saúde geral (SRS) e da saúde oral (SROH) entre pacientes com diabetes melittus tipo 2 (DM2) e sem (nDM2) e explorar as condições de maior impacto na saúde geral (GH) e oral (OH) associadas à autoperceção.
Métodos: Após aprovação ética e consentimento informado, obteve-se 343 adultos DM2 e 323 controlos. Os dados clínicos e de OH foram coletados usando critérios da OMS e perguntas para autoclassificação de GH e OH numa escala de Likert (5 pontos). A análise inferencial utilizou testes não-paramétricos e regressão logística multivariada (MLR) (α=0,05).
Resultados: Os pacientes DM2 autoavaliam significativamente pior a sua GH do que nDM2 (p<0,001). Nos nDM2, a MLR mostrou que cálculo e higiene oral à noite são fatores de risco para uma perceção de pior GH; entre os DM2 é a HTA, dislipidemia, ≥1 dente ausente. Para os DM2, a SRH e SROH, mostra significativamente (p<0,001) mais frequente as perceções de “má” ou “muito má”. O cálculo e bolsas ≥4mm foram identificadas como fatores de risco (OR=3,55, p=0,049; OR=4,32, p=0,025, respetivamente) e CPOD>0, presença de cálculo e recessões estão associadas (p<0,001) a mais frequente perceção como “má” ou “muito má” SRH e SROH.
Conclusões: Indivíduos com diabetes melittus tipo 2 mostraram uma pior autoperceção de saúde geral e oral do que os indivíduos sem esta patologia e a saúde oral foi pior autoavaliada que a saúde geral.
Palavras-chave: Estudo caso-controlo, Cuidados de saúde primários, Autoavaliação da saúde, Autoavaliação da saúde oral, Diabetes tipo 2
Introduction
Oral health (OH) is one of the domains of general health (GH). Both are related to personal well-being, which includes functional capacity, physical wellness, emotional stability, and social-familial interactions.1 Personal well-being also depends on the perception of disease and the way individuals understand several aspects related to health and disease, considering their individual and social experiences, including their knowledge about their pathology,2 symptoms, potential causes, probable duration, evolution in time and potential consequences.3 OH is multi-faceted and includes the abilities to speak, smile, smell, taste, touch, chew, swallow and convey a range of emotions through facial expressions with confidence and without pain, discomfort and any disease of the craniofacial complex.4
Diabetes mellitus is associated with significant multi-morbidity and affects about 422 million individuals worldwide, a number that is increasing. 5, 6 Because diabetes mellitus type 2 (DM2) is a chronic disease, the health status of the affected individuals tends to degrade over time, with the arising of clinical complications mostly due to an increasingly difficult glycaemic control. The development of comorbidities leads to a depreciation of the perceived quality of life.7 Oral pathology is one of the major complications in DM2 patients and, therefore, it is important to intervene early in order to reduce the risk of a future interaction between these pathologies. 8 - 11
The emphasis on similarities between determinants of OH and GH is broadly consistent and leads to the conclusion that OH is an integral part of GH.12 Some studies have identified associations between oral pathology and DM2, 1, 8, 13, 14 and both are associated with a worse perceived GH15 and worse oralhealth quality of life due to OH manifestations. 16, 18
The self-perception of health (SPH) is a subjective indicator that complements the clinical health status, independently of medical interpretations of signs and symptoms, and has been identified as an important predictor of mortality or health care. The SPH is a valid assessment of the individual’s GH and OH perception 19 - 22 and can be used as a predictor of future health outcomes 23, 24 or related to OH in diabetic patients. 25 - 29 The self-perception of impacts on both GH and OH is not always significantly valued in literature, 1, 30 - 32 and it can be theorized that the association between the individuals’ perception of OH and GH is not always clear.33
This study aims to analyze whether there are differences between self-rated general health (SRH) and self-rated oral health (SROH) between individuals with DM2 and without DM2 and to characterize the general and oral conditions associated with general-health and oral-health self-perception.
Material and methods
A case-control study was conducted among adult volunteers with DM2 and without DM2 attending a primary healthcare centre – the Family Health Unit (FHC) of Espinho, Portugal. Ethical clearance (Parecer CES/ARSN n.8/2013) was obtained from the Ethical Board of the Portuguese Northern Regional Health Administration. The permission to examine the patients’ mouth and record the data was obtained from authorities.
Adult volunteers attending the FHC were randomly invited to participate by telephone. For those who accepted, an appointment including oral examination was performed to evaluate if they met the inclusion criteria. Patients with DM2 were invited for the disease group of the study (DM2 group) and healthy volunteers with no DM2 diagnosis for the control group (nDM2 group).34 The pairing mode between cases and controls was partially performed based on gender and age.
A sample size of at least 656 individuals (328 in each group) was calculated for an expected prevalence of dental caries of 65%±5%, an expected difference of 5%, a 95% CI and a power of 90%, based on the III National Study’s results on the prevalence of dental caries in adults.35>
Data on haemoglobin A1c (HbA1c), body mass index (BMI), duration of illness and presence of complications were obtained by consulting the individual electronic or paper clinical process. The clinical analytic data considered was the last one available in the 12 months prior to the interview.
The oral-health status was recorded following the WHO oral health criteria36 through a questionnaire and clinical evaluation.
The questionnaire was administered face-to-face to the volunteers, by asking them to rate their own GH and OH on a Likert 5-point scale (1 – very bad, 2 – bad, 3 – median, 4 – good and 5 – very good).
The oral examination was conducted by a single experienced examiner, who was trained and calibrated (Cohen-Kappa of 0.80 was the minimal value). This examination was done under natural daylight and dental auxiliary light (OSRAM DECOSTAR), using a plain mirror #4 and a periodontal PDT sensor probe. The oral-health status was recorded based on the decayed, missing and filled teeth (DMFT) index and respective components (decayed teeth (DT), missing teeth (MT) and filled teeth (FT)), the Community Periodontal Index (CPI);36 and the use of a prosthesis, if applicable.
Statistical analysis was performed using the IBM© SPSS® Statistics v.25 (IBM© Corporation). The mean values of the nDM2 and DM2 groups were compared using the Student’s independent t-test, while categorical variables were compared using mostly the chi-square test. The binomial test was used to estimate the distribution of the controlled HbA1c value. The relationship of the perceived status of both GH and OH between groups was assessed using chi-square tests. The Bonferroni correction was used for proportion comparisons of more than two categories. The dependent variables of the perceived status of GH and OH were compared between groups based on the median scores of the categories of relevant covariates, using the nonparametric Mann-Whitney’s test or the Kruskal-Wallis test. Whenever the Kruskal-Wallis test showed significant differences, the multiple comparisons were performed considering the Bonferroni correction. The significance level was set at 0.05 for all inferences.
Multivariable binary logistic regression (MLR) models (Wald backward stepwise method, p=0.05 for covariate inclusion and p=0.10 for exclusion) were used to predict associations (as risk or protective factors) between covariables identified in previous analyses and participants having “bad” or “very bad” SRH or SROH. The quality of logistic regressions was evaluated using the area under the curve (AUC), and indicated the adjustment of the model of SRH and SROH to clinical variables.
Results
Most participants were female (56.9%), and the mean age was 63.9±12.8 years old. A significantly (p=0.003) higher percentage of women was in the nDM2 group (62.8%). The BMI higher classes – pre-obesity (47.5%) and obesity (37%), were significantly more frequent (p<0.001) in the DM2 group than in the nDM2. Significantly fewer smokers (7%) and a higher prevalence of clinical comorbidities were found in the DM2 group. A significantly greater part of the DM2 group (p<0.001) were standard 34, 37 controlled DM2 individuals (70%) (Table 1).
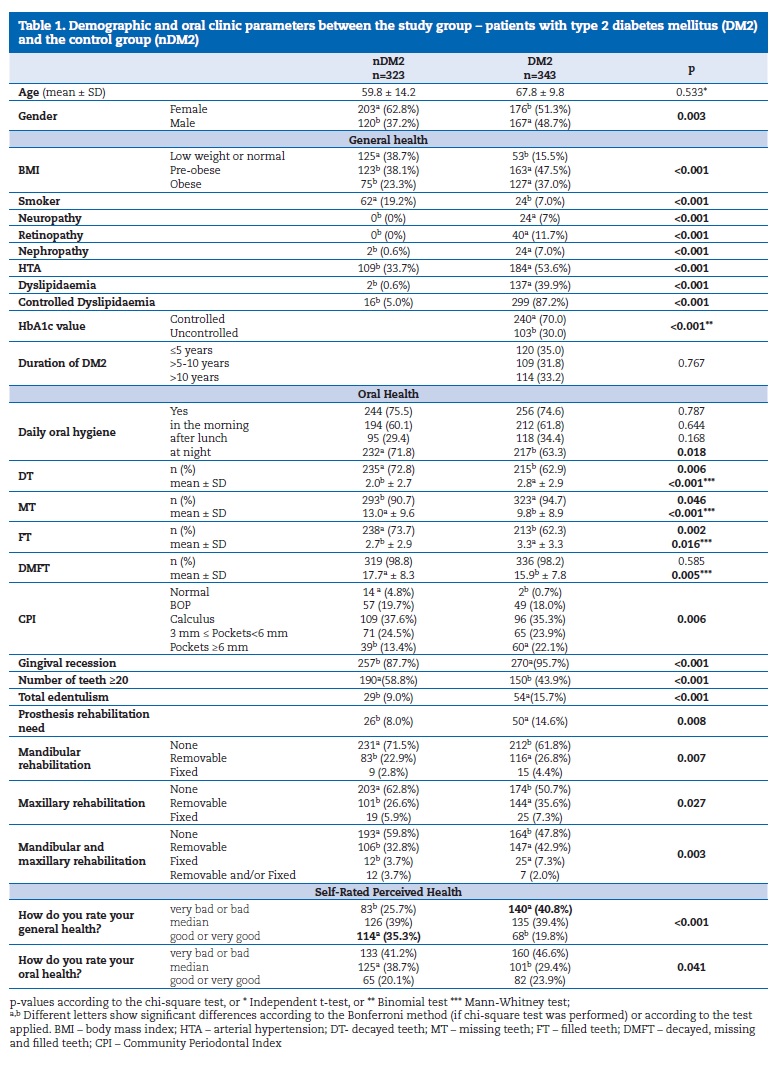
No differences were found in the reported daily oral hygiene between the nDM2 and DM2 groups. The DMFT index was significantly higher (p=0.005) in the nDM2 (17.7±8.3) than in the DM2 group (15.9±7.8), while dental caries experience was not significantly different. The DM2 group had a higher prevalence of periodontal disease (99.3% vs. 95.2% in the nDM2 group, p=0.006), with a higher severity status. The prevalence of periodontal pockets >3mm was 46% for the DM2 and 37.9% for the nDM2 group.
Gingival recession prevalence was 95.7% for the DM2 vs. 87.7% for the nDM2 group (p<0.001). Total edentulism showed a significantly (p<0.001) higher prevalence in DM2 patients, being 1.86 times more prevalent (15.7%) than in the nDM2 group (Table 1).
Regarding the SRH and SROH results, described in Table 1, the DM2 group classified SRH significantly (p<0.001) worse than the nDM2, with “bad” or “very bad” perceptions. The nDM2 participants (Table 2) who rated SRH as “good” or “very good” (59.5%) also did it for SROH. Similarly, 53.1% of the DM2 patients classified both SRH and SROH as “very bad” or “bad.” These results show a significant agreement in these classifications.
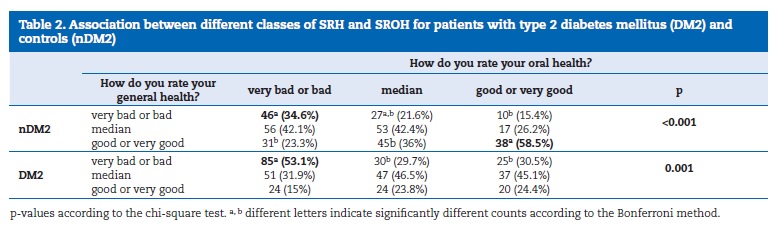
Table 3 reports the association between median and interquartile range and the socio-demographic and clinical variables.
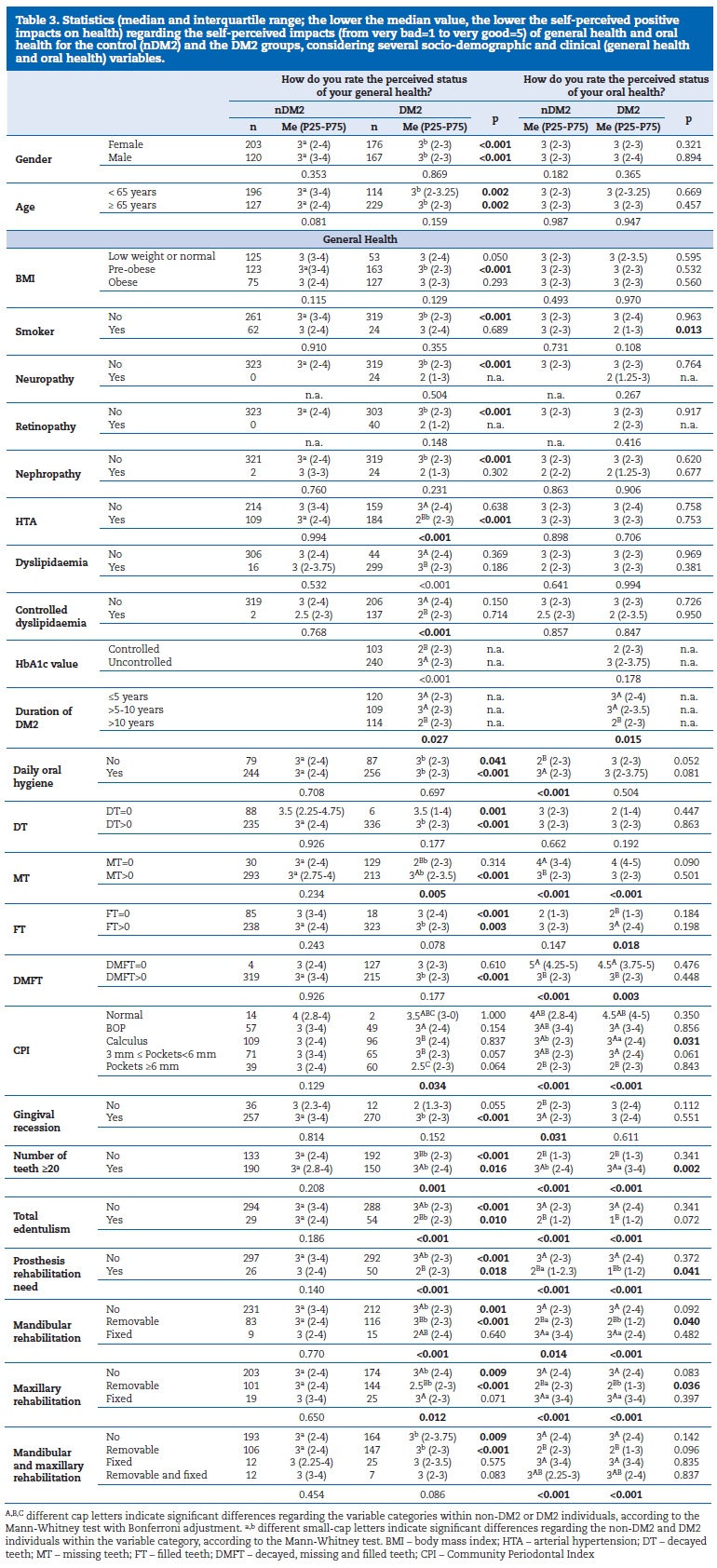
The higher the median value, the higher the perception of positive impacts on GH and OH for the DM2 and nDM2 groups. Although this was a rare condition, almost all DM2 and nDM2 participants with no caries experience (DMFT=0) classified their OH status as “good” or “very good,” with only 1% of these DM2 patients classifying their OH as “median.”
MLR identified variables significantly and independently associated with “bad” or “very bad” SRH or SROH in both groups (Table 4). In the nDM2 group, “bad” or “very bad” SRH was not associated with any GH clinical variable, while the CPI was found to be a risk factor for that outcome and having calculus was a significant risk factor of “bad” health (OR=3.21, 95%CL:1.03-9.99; p=0.044). In the DM2 group, the CPI was also found to be a risk factor for”bad” or “very bad” SRH (p=0.015), and arterial hypertension (HTA) and dyslipidaemia were other significant risk factors (ORHTA=16.62, p<0.001 and ORDysl=5.17, p<0.001).
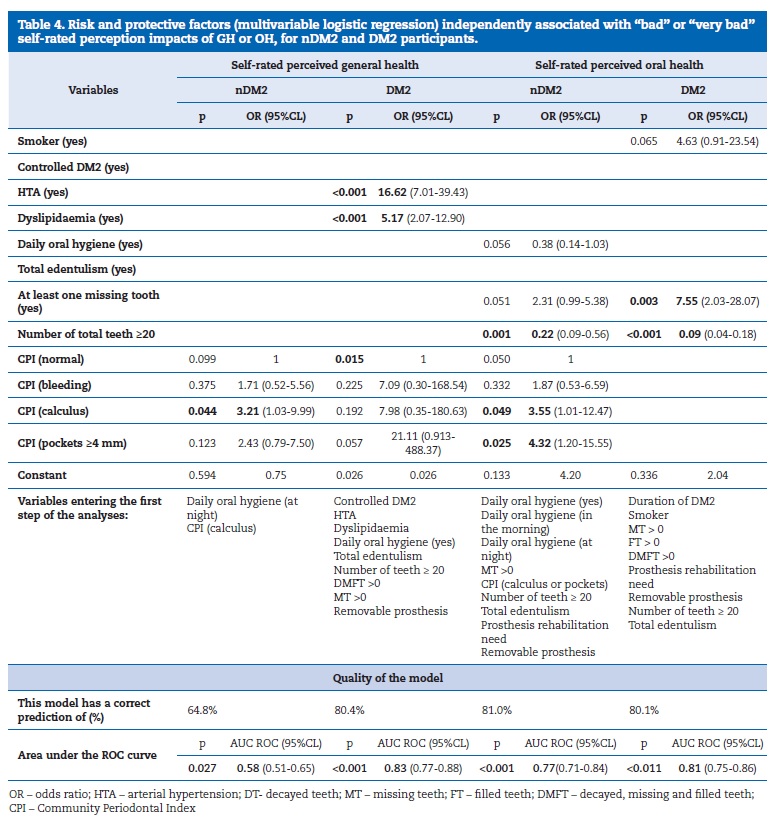
Regarding the “bad” or “very bad” SROH, the CPI was identified as a risk factor in the nDM2 group, together with calculus and pockets >4mm (OR=3.55, p=0.049, and OR=4.32, p=0.025, respectively). On the other hand, having at least 20 teeth was found to be a protective factor (OR=0.22, p=0.001), decreasing the chance of that outcome in 78%. In the DM2 group, having at least one tooth missing was a risk factor for “bad” or “very bad” SROH (OR=7.55, p=0.003) while having at least 20 teeth was a protective factor (OR=0.09, p<0.001), decreasing the chance of that outcome in 91%.
The AUC values obtained for some models (AUC higher than 80%, p<0.010) showed a good adjustment to predict a “bad” or “very bad” SRH in the DM2 group and SROH in the nDM2 and DM2 groups. Thus, a potential clinical application of these predictive models can be considered. On the other hand, that cannot be said for SRH in the nDM2 group, which showed a reasonable adjustment (AUC =~65%, p=0.027) (Table 4).
Discussion
The present study confirms the hypothesis that SRH and SROH were perceived differently by DM2 and nDM2 participants.
The DM2 group had worse SRH and SROH than the nDM2, and OH was generally self-rated worse than GH regardless of the presence of DM2.
The sample showed a significantly higher frequency of women in the nDM2 group and men in the DM2 group, which is similar to the study by Liu,38 and may be justified by women being more likely to go to health centres than men. Like in other studies,39,40 the DM2 group had significantly more participants in the heavier classes of BMI than the nDM2 group.
However, no relation was found between MT and obesity, as described by Nascimento.41 Regarding SRH, the nDM2 participants with a pre-obese condition presented better health perception than DM2 participants. Similarly to other studies, 8, 42 the DM2 group had significantly fewer smokers, and tobacco use was significantly different among the DM2 participants, probably due to a perception of its negative impacts on OH.
There were no significant differences in daily oral hygiene habits between groups. As reported by other authors, the periodontal condition was significantly better in the nDM2 than in the DM2 group. 13, 14, 38 The most severe forms of periodontitis in DM2 participants, with more gingival recession and periodontal pockets >3mm, had a more negative impact on SRH and SROH.
Regarding associations between SRH and SROH, a better concordance was found in the nDM2 group, whereas DM2 participants reported significantly more negative impacts by rating GH and OH as “very bad” and “bad” more often. As theorized by Petersen33 and reported in other studies, 1, 30 - 32, 44 DM2 patients showed a poorer perception of health than nDM2 individuals, which explains why the “median” OH classification was significantly more reported in controls than in DM2 patients. The same goes for the opposite extreme of perception, as nDM2 participants rated OH and GH as “good” or “very good” more often. Regarding the socio-demographic variables, there were no significant differences in SRH and SROH.
Individuals with an HbA1c value indicating a lack of control of DM2 showed better SRH, probably because they did not value their health status adequately as they did not feel the complications of DM2 significantly affecting them. The other DM2 complications probably justified a worse self-perception of GH and OH by those who had an older diagnosis comparing to those with up to 10 years of disease.
DM2 patients with HTA or dyslipidaemia showed worse SRH, while those who had normotension showed a significantly better perception of GH. None of these systemic characteristics revealed a significantly different impact on SROH between groups.
Regarding OH variables, including daily oral hygiene, DT>0, MT>0, FT, DMFT>0, <20 teeth in the mouth, total edentulism and need of prosthesis rehabilitation, the latter was associated with a higher perception of GH as “bad” or “very bad” in DM2 patients. Within the DM2 group, a severe CPI classification, <20 teeth or total edentulism and no prosthesis rehabilitation were associated with a worse perception of GH. These data support the concept of OH influencing GH and the quality of life of individuals. 1, 3, 12, 22, 24
The absence of daily oral hygiene habits and calculus showed more negative impacts only among nDM2 participants.
However, the presence of caries lesions was not significant. Calculus (had lower medians than the “normal” state), MT>0 and DMFT>0 showed significantly negative impacts in both groups, and FT>0 only in the DM2 group. Total edentulism, the absence of fewer than 20 teeth and the need of prosthetic rehabilitation had negative impacts with a significantly worse perception of OH. Oral rehabilitation is always related to removable prostheses (independently of being maxillary, mandibular or both), and these showed a more negative impact on GH and OH.
The MLR showed that the factors with greater relevance for a worse perceived GH were the presence of calculus and oral hygiene at night for the nDM2 group. As for the DM2 group, those factors were HTA, dyslipidaemia, total edentulism and at least one missing tooth, although this was a protective factor (OR=0.45, IC95%: 0.20-0.99) when DM2 was controlled.
Regarding SROH in the nDM2 group, the MLR showed that having at least 20 teeth represented a protective factor (OR=0.22, IC95%:0.089-0.556) and the presence of calculus or a periodontal pocket >3.5mm had a negative impact. Daily oral hygiene “in the morning” or “at night”, MT>0 and total edentulism, when included in the model, were found to be predictor factors for a negative impact on OH in nDM2 participants.
Finally, the MLR showed that the predictors of a negative SROH in DM2 patients were: the duration of DM2, smoking habits, MT>0, FT>0, DMFT> 0, total teeth <20 and total edentulism.
This estimated model has a correct prediction of 80.1%. The fact that the pairing was performed only from an analytical perspective and not by pairing each participant could be a limitation of the study. However, as Pierce44 pointed out in his methodological review article, when there are no problems with data dispersion, a standardized statistical analysis for a case-control study can be performed.
Because this study is based on a clinical sample obtained in a primary public health centre that follows the most diabetic patients for disease monitoring in Portugal, the results should be generalized to the overall population with care.
Conclusions
The present study confirms the hypothesis that DM2 patients had a worse SRH and SROH than individuals without DM2. Both groups perceived GH and OH differently, and OH was generally self-rated worse than GH regardless of having DM2.
The prevalence of oral pathologies in both groups was high. However, DM2 patients presented fewer caries lesions, more extracted teeth, more FT, and few were rehabilitated.
The presence of more advanced forms of periodontal disease (pockets) affected the SRH and SROH negatively. Simultaneously, an older diagnosis of DM2 and tobacco use induced the greater perception of negative impacts on OH among DM2 patients.
The most developed primary health centres should include access to oral care in order to monitor OH regularly, as well as other macro and microvascular complications in DM2 patients.
Better attention centred in the OH patient by healthcare units is needed, and the access to OH professionals could be a way of improving diabetic patient’s GH and OH.
References
1. Irani FC, Wassall RR, Preshaw PM. Impact of periodontal status on oral health-related quality of life in patients with and without type 2 diabetes. J Dent. 2015;43:506-11.
2. Cohen G, Forbes J, Garraway M. Interpreting self-reported limiting long term illness. Br Med J. 1995;311:722-4.
3. Baiju R, Peter E, Varghese NO, Sivaram R. Oral health and quality of life: current concepts. J Clin Diagn Res. 2017;11:ZE21-6.
4. Glick M, Williams DM, Kleinman DV, Vujicic M, Watt RG, Weyant RJ. A new definition for oral health developed by the FDI World Dental Federation opens the door to a universal definition of oral health. Int Dent J. 2016;66:322-4.
5. WHO. Global report on diabetes. WHO ed. 2016. Geneve.
6. Chapple IL, Bouchard P., Cagetti MG, Campus G, Carra MC, Cocco F, et al. Interaction of lifestyle, behaviour or systemic diseases with dental caries and periodontal diseases: consensus report of group 2 of the joint EFP/ORCA workshop on the boundaries between caries and periodontal diseases. J Clin Periodontol. 2017;44(Suppl 18):S39-51.
7. Sousa VD, Zauszniewski JA, Musil CM, Price-Lea PJ, Davis, SA. Relationships among self-care agency, self-efficacy, self-care, and glycemic control. Res Theory Nurs Pract. 2005;19:217-30.
8. Mohamed HG, Idris SB, Ahmed MF, Bøe OE, Mustafa K, Ibrahim SO, Astrøm AN. Association between oral health status and type 2 diabetes mellitus among Sudanese adults: a matched case-control study. PLoS One. 2013;8:e82158.
9. Bissong M, Azodo CC, Agbor MA, Nkuo-Akenji T, Fon PN. Oral health status of diabetes mellitus patients in Southwest Cameroon. Odontostomatol Trop. 2015;38:49-57.
10. Trentin MS, Verardi G, De C Ferreira M, de Carli JP, da Silva SO, Lima IF, et al. Most Frequent Oral Lesions in Patients with Type 2 Diabetes Mellitus. J Contemp Dent Pract. 2017;18:107-11.
11. Mauri-Obradors E, Estrugo-Devesa A, Jané-Salas E, Viñas M, López-López J. Oral manifestations of Diabetes Mellitus. A systematic review. Med Oral Patol Oral Cir Bucal. 2017;22:e586-94.
12. Sheiham A. Oral health, general health and quality of life. Bull World Health Organ. 2005;83:644.
13. Taylor GW, Manz MC, Borgnakke WS. Diabetes, periodontal diseases, dental caries, and tooth loss: a review of the literature. Compend Contin Educ Dent 2004;25:179-92.
14. Kim EK, Lee SG, Choi YH, Won KC, Moon JS, Merchant AT, et al. Association between diabetes-related factors and clinical periodontal parameters in type-2 diabetes mellitus. BMC Oral Health. 2013;13:64.
15. Edelman D, Olsen MK, Dudley TK, Harris AC, Oddone EZ. Impact of diabetes screening on quality of life. Diabetes Care. 2002;25:1022-6.
16. Drumond-Santana T, Costa FO, Zenóbio EG, Soares RV, Santana TD. Impacto da doença periodontal na qualidade de vida de indivíduos diabéticos dentados. Cad Saude Publica. 2007;23:637-44.
17. Araújo AC, Gusmão ES, Batista JE, Cimões R. Impact of periodontal disease on quality of life. Quintessence Int. 2010;41:e111-8.
18. Mourão LC, Garcia E, Passos D, Lorena T, Canabarro A. Impact of well-controlled type 2 diabetes mellitus on quality of life of chronic periodontitis patients. J Indian Soc Periodontol. 2016;20:623-6.
19. Alonzo AA. Everyday illness behavior: a situational approach to health status deviations. Soc Sci Med. 1979;13A:397-404.
20. Vintém JM. Inquéritos nacionais de saúde: Auto-percepção do estado de saúde: Uma análise em torno da questão de género e da escolaridade. Rev Saúde Pub. 2008;6:5-18.
21. Locker D, Matear D, Stephens M, Jokovic A. Oral health-related quality of life of a population of medically compromised elderly people. Community Dent Health. 2002;19:90-7.
22. Kieffer JM, Hoogstraten J. Linking oral health, general health, and quality of life. Eur J Oral Sci. 2008;116:445-50.
23. Laursen DH, Christensen KB, Christensen U, Frølich A. Self-rated health as a predictor of outcomes of type 2 diabetes patient education programmes in Denmark. Public Health 2016; 139: 170-7.
24. Benyamini Y, Leventhal H, Leventhal EA. Self-rated oral health as an independent predictor of self-rated general health, self-esteem and life satisfaction. Soc Sci Med. 2004;59:1109-16.
25. Maia FB, de Sousa ET, Sampaio FC, Freitas CH, Forte FD. Tooth loss in middle-aged adults with diabetes and hypertension: Social determinants, health perceptions, oral impact on daily performance (OIDP) and treatment need. Med Oral Patol Oral Cir Bucal. 2018;23:e203-10.
26. Sandberg GE, Sundberg HE, Wikblad KF. A controlled study of oral self-care and self-perceived oral health in type 2 diabetic patients. Acta Odontol Scand. 2001;59:28-33.
27. Sabbah W, Tsakos G, Chandola T, Sheiham A. Watt RG.Social gradients in oral and general health. J Dent Res. 2007;86:992-6.
28. Cinar AB, Oktay I, Schou L. Self-efficacy perspective on oral health behaviour and diabetes management. Oral Health Prev Dent. 2012;10:379-87.
29. Shanmukappa SM, Nadig P, Puttannavar R, Ambareen Z, Gowda TM, Mehta DS. Knowledge, Attitude, and Awareness among Diabetic Patients in Davangere about the association between diabetes and periodontal disease. J Int Soc Prev Community Dent. 2017;7:381-8.
30. Durham J, Fraser HM, McCracken GI, Stone KM, John MT, Preshaw PM. Impact of periodontitis on oral health-related quality of life. J Dent. 2013;41:370-6.
31. Buset SL, Walter C, Friedmann A, Weiger R, Borgnakke WS, Zitzmann NU. Are periodontal diseases really silent? A systematic review of their effect on quality of life. J Clin Periodontol. 2016;43:333-44.
32. Corrêa K, Gouvêa GR, Silva MA, Possobon RF, Barbosa LF, Pereira AC, et al. Quality of life and characteristics of diabetic patients. Cien Saude Colet. 2017;22:921-30.
33. Petersen PE. Global policy for improvement of oral health in the 21st century–implications to oral health research of World Health Assembly 2007, World Health Organization. Community Dent Oral Epidemiol. 2009;37:1-8.
34. DGS. Diagnóstico e Classificação da Diabetes Mellitus. Norma n.º 002/2011 de 14/01/2011. DGS ed. 2011. Lisboa.
35. DGS. III Estudo Epidemiológico Nacional das Doenças Orais. DGS ed. 2015. Lisboa.
36. WHO. Oral Health Surveys-Basic Methods. 5th ed. 2013.Geneve.
37. DGS. Prescrição e determinação da Hemoglobina Glicada A1c. Norma n.º 033/2011 de 30/9/2011 e actualizada a 6/02/2012. DGS ed. 2011. Lisboa.
38. Liu Y, Yu Y, Nickel JC, Iwasaki LR, Duan P, Simmer-Beck M, et al. Gender differences in the association of periodontitis and type 2 diabetes. Int Dent J. 2018;68:433-40.
39. Isabel CA, Moysés MR, van der Bilt A, Gameiro GH, Ribeiro JC, Pereira LJ. The relationship between masticatory and swallowing behaviors and body weight. Physiol Behav. 2015;151:314-9.
40. Flores-Orozco EI, Tiznado-Orozco GE, Osuna-González OD, Amaro-Navarrete CL, Rovira-Lastra B, Martinez-Gomis J. Lack of relationship between masticatory performance and nutritional status in adults with natural dentition. Arch Oral Biol. 2016;71:117-21.
41. Nascimento GG, Leite FR, Conceição DA, Ferrúa CP, Singh A, Demarco FF. Is there a relationship between obesity and tooth loss and edentulism? A systematic review and meta-analysis. Obes Rev. 2016;17:587-98.
42. Javed F, Al-Kheraif AA, Salazar-Lazo K, Yanez-Fontenla V Aldosary KM, Alshehri M, Malmstrom H, Romanos GE. Periodontal inflammatory conditions among smokers and never-smokers with and without type 2 diabetes mellitus. J Periodontol. 2015;86:839-46.
43. Cinar AB, Oktay I, Schou L. Toothbrushing: A link between non-communicable and communicable diseases? Oral Health Prev Dent. 2015;13:515-22.
44. Pierce N. Analysis of matched case-control studies. BMJ. 2016;352:i969.
M. Conceicao Manso
Correio eletronico: cmanso@ufp.edu.pt
Ethical disclosures
Protection of human and animal subjects. The authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of data. The authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consent. The authors declare that no patient data appear in this article.
Conflict of interest
The authors have no conflicts of interest to declare.
Acknowledgements
J. Frias-Bulhosa acknowledges the coordinator of USF Espinho, Dr. Jorge Vinagre, Dr. Helena Beça, who was the main interlocutor, and all the other professionals involved for their collaboration during the data collection for this study.
Article history:
Received 9 June 2018
Accepted 27 December 2018
Available online 6 February 2019
