
Revista Portuguesa de Estomatologia, Medicina Dentária e Cirurgia Maxilofacial
SPEMD - Rev Port Estomatol Med Dent Cir Maxilofac | 2018 | 59 (3) | 154-161
Original research
Chlorhexidine loading of acrylic reline resins – Microhardness and flexural strength after thermal aging
Incorporação de clorexidina em resinas acrílicas de rebasamento – Microdureza e resistência à flexão após envelhecimento térmico
a Faculdade de Medicina Dentária, Universidade de Lisboa, Lisboa, Portugal
b Faculdade de Farmácia, Universidade de Lisboa, Lisboa, Portugal
Cristina Bettencourt Neves - cristina.neves@fmd.ulisboa.pt
Article Info
Rev Port Estomatol Med Dent Cir Maxilofac
Volume - 59
Issue - 3
Original research
Pages - 154-161
Go to Volume
Article History
Received on 08/08/2018
Accepted on 18/11/2018
Available Online on 29/11/2018
Keywords
Original research
Chlorhexidine loading of acrylic reline resins – Microhardness and flexural strength after thermal aging
Incorporação de clorexidina em resinas acrílicas de rebasamento – Microdureza e resistência à flexão após envelhecimento térmico
Inês Rijoa, Daniel Pedroa, Joana Costaa, Ana Bettencourtb, Jaime Portugala, Cristina Bettencourt Nevesa,*
a Faculdade de Medicina Dentária, Universidade de Lisboa, Lisboa, Portugal
b Faculdade de Farmácia, Universidade de Lisboa, Lisboa, Portugal
http://doi.org/10.24873/j.rpemd.2018.11.237
Abstract
Objectives: To evaluate the effect of chlorhexidine loading in microhardness and flexural strength of acrylic reline resins, after thermal aging.
Methods: Several concentrations of chlorhexidine were selected to load three acrylic reline resins: 1%, 2.5%, 5%, and 7.5% in Kooliner, 1%, 2.5%, 5%, 7.5% and 10% in Ufi Gel Hard and 1%, 2.5% and 5% in Probase Cold. In the control group the reline resin was not loaded with chlorhexidine. Eight specimens per group (n=8) were fabricated (64x10x3.3 mm) and submitted to thermal aging (1000 cycles, 5ºC-55ºC). Knoop microhardness (30 s, 98 mN) and 3-point flexural strength (5 mm/min) tests were performed in each specimen. Results were submitted to nonparametric tests Kruskall-Wallis e Mann-Whitney (α=0.05).
Results: No differences (p>0,05) were found among microhardness of Kooliner and Probase Cold loaded with the tested chlorhexidine concentrations. Ufi Gel Hard with 2.5% of chlorhexidine yielded higher (p<0,05) microhardness than the control and 7.5% chlorhexidine groups. Regarding to flexural strength, no differences (p>0,05) were observed for Kooliner and Ufi Gel Hard. Loading Probase Cold with 5% of chlorhexidine lead to lower (p=0.033) flexural strength values than control group.
Conclusions: Loading Kooliner and Ufi Gel Hard with the studied concentrations of chlorhexidine does not negatively affect microhardness or flexural strength, after thermal aging. Loading Probase Cold with 5% of chlorhexidine does not affect microhardness, but leads to a decrease of the flexural strength.
Keywords: Chlorhexidinem, Denture relining, Denture stomatitis, Flexural strength, Hardness
Resumo
Objetivos: Avaliar o efeito da incorporação de clorexidina na microdureza e resistência à flexão de resinas acrílicas de rebasamento, após envelhecimento térmico.
Métodos: Foram selecionadas diversas concentrações de clorexidina e incorporadas em três resinas acrílicas de rebasamento: 1%, 2,5%, 5% e 7,5% em Kooliner; 1%, 2,5%, 5%, 7,5% e 10% em Ufi Gel Hard; e 1%, 2,5% e 5% em Probase Cold. No grupo de controlo a resina não foi incorporada com clorexidina. Oito espécimes por grupo (n=8) foram fabricados (64x10x3,3 mm) e submetidos a envelhecimento térmico (1000 ciclos, 5ºC-55ºC). Foram realizados testes de microdureza Knoop (30 s, 98 mN) e de resistência à flexão a três pontos (5 mm/min). Os dados obtidos foram submetidos a testes não-paramétricos Kruskall-Wallis e Mann-Whitney (α=0,05).
Resultados: Não se encontraram diferenças (p>0,05) entre a microdureza da Kooliner e Probase Cold incorporada com as diversas concentrações de clorexidina estudadas. A resina Ufi Gel Hard com 2,5% de clorexidina permitiu atingir valores de microdureza superiores (p<0,05) relativamente aos grupos controlo e com 7,5% de clorexidina. Relativamente à resistência à flexão, não foram encontradas diferenças (p>0,05) nas resinas Kooliner e Ufi Gel Hard. A incorporação de Probase Cold com 5% de clorexidina conduziu a valores de resistência à flexão inferiores (p= 0,033) ao controlo.
Conclusões: A incorporação das concentrações estudadas de clorexidina nas resinas Kooliner e Probase Cold não afetou negativamente a microdureza ou a resistência à flexão, após envelhecimento térmico. O carregamento do Probase Cold com 5% de clorexidina não influenciou a microdureza, mas provocou a diminuição da resistência à flexão.
Palavras-chave: Clorexidina, Rebasamento, Estomatite protética, Resistência à flexão, Dureza.
Introduction
Residual ridge resorption is a chronic and progressive phenomenon of bone remodeling that decreases denture stability and retention, reducing the comfort of patients wearing a removable prosthesis.1 A poor fit denture can cause mucosal trauma which, associated with other factors such as poor oral hygiene,2 dietary factors,3, 4 xerostomia,5 absence of overnight removal,6, 7 chronic diseases or a compromised immune system,7 can lead to denture‑induced stomatitis.
A reline procedure is commonly used to enhance the fit of the pre‑existing denture to hard and soft tissues, and auto‑polymerizing acrylic reline resins are usually the chosen material to perform this relatively simple, useful and inexpensive treatment. 8, 9 Either direct resins, that are cured at the chairside in the dental clinic, or indirect resins, which are cured atthe laboratory, can be used.
Acrylic resins are porous materials susceptible to oral biodegradation and mechanical surface deterioration.10, 11 The roughness and irregularity of the resulting surface may act as reservoirs of microorganisms, which in turn may contribute to oral diseases. 12 ‑ 15
The main pathogen related to denture stomatitis is Candida albicans due to its ability to adhere and proliferate through tissues of the oral cavity and acrylic resins producing a complex and heterogeneous bacterial biofilm. 15, 16
Candida‑associated denture stomatitis is the most common form of oral candidiasis, affecting mostly the palatal mucosa. 17 Its clinical appearance can be a discrete area of pinpoint inflammation related to the ducts of the palatal mucous glands or an intense erythematous area of the mucosa covered by the denture. 16, 18 Even though this oral disease is usually asymptomatic, it should be treated as it can progress to more severe infections. 19
The treatment of Candida‑associated denture stomatitis is usually based on cleansing,20 relining or even replacement of the denture, together with the prescription of antifungal drugs.9, 21
The topical application of an antifungal agent can be highly inefficient due to the rapid drug clearance from the site of infection.
Moreover, it is challenging to obtain rigid patient compliance and, when drugs are given systemically, only a small concentration of the drug tends to reach the target location, associated to an increased risk of undesired side effects. 22, ‑ 24
Besides that, even when additional hygiene solutions are used for denture cleansing, Candida strains tend to subsist. 20, 25, 26
The feasibility of introducing antimicrobial and antifungal agents in resins, acting as drug carriers for the treatment of denture‑induced stomatitis, has been investigated by several researchers.24 ‑ 28 These drug delivery systems have some advantages, such as preservation of therapeutic levels by continuous drug release at the infection site, minimal risk of systemic toxicity, decrease need of patient compliance and, when incorporated in reline materials, simultaneous treatment of ill‑fitting dentures and Candida‑related infection. 22, 29 ‑ 31
Furthermore, with the use of these carriers, the effect of preventing the initial adhesion of microorganisms to the base of the denture and inhibition of biofilm formation is added, resulting in an important interference in the mechanism of infection. 24, 26, 29
Chlorhexidine (CHX) is a widely used antiseptic drug with remarkable antifungal, antibacterial and anti‑biofilm abilities, and a high substantivity. 23, 26, 32 CHX suppress the adherence of Candida albicans to cells or acrylic surfaces and, for this reason, can inhibit Candida‑related infections. 27, 33 ‑ 37 CHX has shown to have a good performance both on releasing and microbiological tests. 22, 35, 38 ‑ 42 When loaded into acrylic resins, CHX has shown higher effectiveness in microbiological tests compared to other agents such as fluconazole and nystatin. 23, 24, 36, 42 Also, releasing rates with CHX loaded acrylic resins showed a pattern of high CHX release at the first two to seven days, that decrease and became steadier for twenty‑eight days. 36, 38, 43
The concentration of 10% of CHX is usually accepted as the most effective against Candida albicans. 39 ‑ 41 However, the minimal concentration of CHX (w/w) to load into reline acrylic resins with proper antifungal activity against Candida albicans seems to be 2.5% for Kooliner, and 5% for Ufi Gel Hard and Probase Cold. 34
Previous studies44, 45 showed promising results since loading acrylic reline resins with antifungal CHX concentrations did not influence mechanical properties. However, in that studies, the mechanical tests were performed a short period after polymerization, remaining some concerns about the long‑term effect of CHX loading on mechanical and surface properties, that can occur when the acrylic resin is in function and submitted to the intraoral environment.10, 46 ‑ 49
The objective of this study was to evaluate the effect of CHX loading in microhardness and flexural strength of acrylic reline resins, after thermal aging, according to the following hypotheses: loading 1) Kooliner, 2) Ufi Gel Hard, or 3) Probase Cold with different concentrations of CHX does not affect the microhardness and the flexural strength values of the reline acrylic resin.
Materials and methods
Three auto‑polymerizing acrylic resins presented in the powder‑liquid form were used (Table 1). Two of them are direct reline resins, Kooliner and Ufi Gel Hard, mainly composed by pre‑polymerized poly(ethyl methacrylate) particles.
The third material is an indirect reline resin, Probase Cold, mainly formed by pre‑polymerized poly(methyl methacrylate) particles.50
The acrylic resins were manipulated according to the respective manufacturer’s instructions (Table 1). The liquid was measured using a pipette, and the powder was weighed using a precision scale (Mettler Toledo). All specimens were loaded with chlorhexidine diacetate monohydrate (Panreac Applichem, Darmstadt, Germany) using a mortar and pestle for homogenization, according to the proportions previously established: Kooliner: 0%, 1%, 2.5%, 5% and 7.5% of the acrylic resins’ powder weight (w/w); Ufi Gel Hard: 0%, 1%, 2.5%, 5%, 7.5% and 10% of the acrylic resins’ powder weight (w/w); Probase Cold: 0%, 1%, 2.5 % and 5% of the acrylic resins’ powder weight (w/w).
Rectangular shaped stainless‑steel molds were used to prepare specimens of final dimensions correspondent to 64x10x3.3 mm.51 The stainless‑steel mold was placed on a glass plate covered by a polyester sheet, and the material’ dough was prepared and placed into the mold. After that, another polyester sheet and glass were positioned on top of the mold, and the specimen was kept under compression, at 37±2 ºC, until the end of the set time established by the manufacturer (Table 1). On the other hand, polymerization of the indirect reline resin was carried out in a pressure device (Ivomat, Ivoclar Vivadent, Liechtenstein) at the recommended time, temperature and pressure (Table 1).
After polymerization, the samples were removed from the molds, and the edges of each sample were polished with a 600‑grit silicon carbide paper (Carbimet Paper Discs, Buehler Ltd., IL, USA), on a polisher with constant refrigeration.
Eight specimens of each group (n=8) were prepared, in a total of one hundred eighteen specimens. Control group, with no CHX loaded, was represented as the 0% CHX group. 52
All specimens were submitted to a thermal aging process, being exposed to 1000 cycles of thermal fluctuations between 5 ºC and 55 ºC, immersing the specimens for 20 seconds in each water bath (5 seconds of dwell time; Refri 200‑E, Aralab, Cascais, Portugal).
The Knoop microhardness test was performed (Duramin, Struers DK 2750, Ballerup, Denmark) with a 98.12 mN load for 30 seconds. Twelve equidistant measurements were made in each specimen, and the mean value was used as the Knoop microhardness (KHN) of the specimen.
After checking the width and thickness of each specimen with a digital micrometer of 0.01 mm precision (Mitutoyo Digimatic, MFG. Co. Ltd, Tokyo, Japan), flexural strength was tested with a universal testing machine (Instron Model 4502, England), using a three‑point bending device with a distance between supports of 50 mm and 1 kN load cell at a crosshead speed of 5 mm/min.51
Since normality was not verified (Shapiro‑Wilk normality tests, p<0.05), data were submitted to Kruskal‑Wallis nonparametric tests, followed by multiple comparisons using Mann‑Whitney tests with Bonferroni corrections (α=0.05).
Results
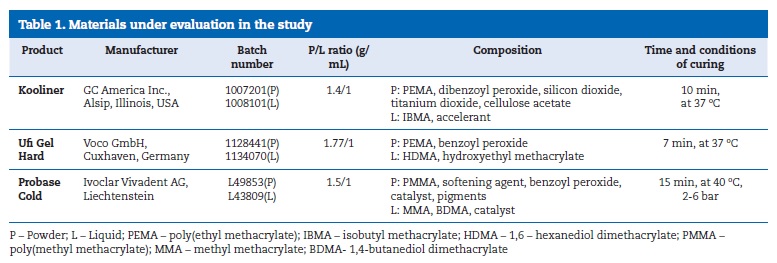
The microhardness of Kooliner was not significantly (p>0.05) affected by the studied percentages of CHX (Figure 1). However, loading Ufi Gel Hard with CHX showed a statistical (p=0.002) influence on the KHN of this direct reline resin.
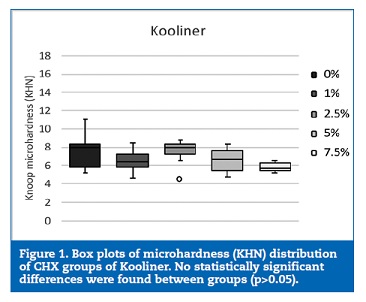
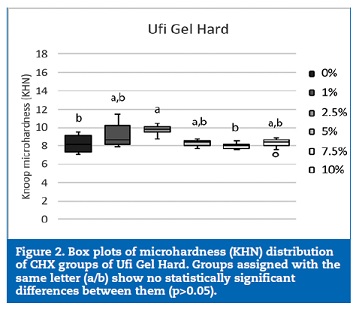
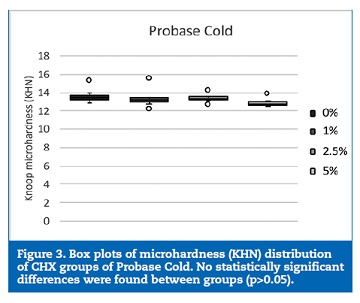
The 2.5% CHX group showed higher KHN values than the control (p=0.042) and 7.5% CHX (p=0.002) groups (Figure 2). No statistically significant (p>0.05) differences were found between the groups of specimens fabricated with Probase Cold (Figure 3).
Regarding flexural strength, neither Kooliner nor Ufi Gel Hard was significantly (p>0.05) affected by CHX loading (Figures 4 and 5). However, loading Probase Cold with 5% of CHX resulted in a statistically (p=0.033) lower flexural strength than the control group. No other significant (p>0.05) differences were found between the flexural strength observed in the Probase Cold groups (Figure 6).
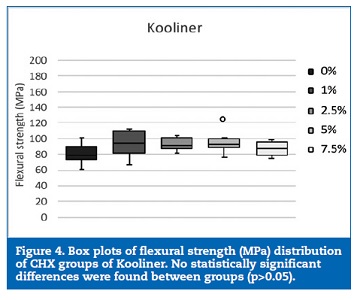
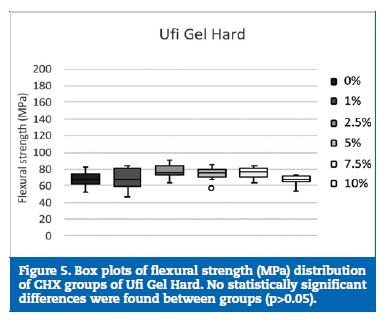
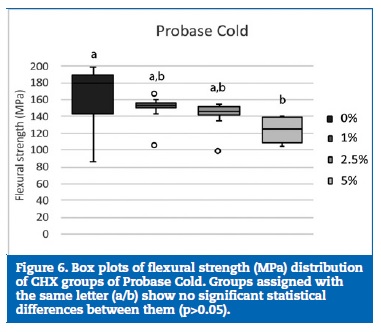
Discussion
In the present study, Koop microhardness and three‑point flexural tests were performed to evaluate the long‑term effect of CHX loading on mechanical and surface properties of different acrylic reline resins.
In spite of incorporation of resin materials, with compounds like fluconazole, silver‑zinc zeolite, fluoroalkyl methacrylate, methacryloyloxyundecylpyridinium bromide or TiO2 and SiO2 nanoparticles, have been studied, 15, 48, 53 CHX has shown a more efficient candidacidal effect when loaded in acrylic resins.22 ‑ 24, 27, 33, 37, 42, The selection of the tested CHX concentrations was based on the results of previous mechanical studies.44 45 Unlike Ufi Gel Hard, loading the Kooliner and the Probase Cold with 10% CHX negatively influences microhardness and flexural strength of these reline resins.44 Likewise, a negative impact on the flexural strength of Probase Cold when loaded with 7.5% CHX was shown.45
The three reline resins were chosen due to their differences in chemical composition and structural arrangement.9 The Kooliner is composed by the isobutyl methacrylate monomer and forms a simple non‑crosslinking net when polymerization is complete. Whereas, the Ufi Gel Hard has the monomer 1,6‑hexanodioldimethacrylate and forms a more complex crosslinking net, with large molecules. The Probase Cold is a methyl methacrylate based resin, that is polymerized in laboratory developing a net with a reduced percentage of uncured monomer. 50, 54 Since these resins have different physical structure and chemical composition, CHX molecules when incorporated in the net can create different links to the polymeric chains and change their properties in distinct magnitudes. Also, CHX incorporation can increase the distance betwee polymer molecules, resulting in an expected weaker polymer net.
The Knoop microhardness test is commonly used in polymeric materials such as acrylic resins because, due to the geometry of the indenter, it minimizes the elastic recovery of these materials.9, 55 Flexural strength was selected since it is one of the most significant features when assessing dental polymers and it gives good information about the clinical performance of the denture when subjected to mastication forces.9,15,56,57
The physical and mechanical properties of the CHX loaded‑acrylic reline resins under study were already investigated.44, 45, 58 Nevertheless, these studies focused on measuring the properties of the resins without any influence of the environment.
So, another feature of the present study was to submit the specimens to a thermal aging procedure, to simulate temperature fluctuations that occur in the oral cavity with mouth breathing and whenever food and beverages with different temperatures are consumed. 59 The specimens were submitted to 1000 cycles of thermal fluctuations between 5 oC and 55 oC, corresponding to 6 weeks in function.60 Most of the CHX loaded in acrylic resins is released into the oral environment in the first twenty‑eight days. 22, 42, 43, 61 However, although after this period the therapeutic dose of CHX gradually becomes less efficient, the reline materials tested are considered to be semi‑permanent and therefore it is important to know whether the loaded acrylic resins maintain their mechanical properties even after the release of CHX.
In the present study, loading the reline acrylic resin with CHX had a dissimilar effect on the microhardness and flexural strength among the three reline materials tested.
Since no statistically significant differences were found between Kooliner groups with different CHX loading, in both microhardness and flexural strength, the first hypothesis in study could not be rejected. However, loading the reline resin with CHX did affect the microhardness of Ufi Gel Hard and the flexural strength of Probase Cold. As so, the second and third hypothesis could be rejected.
However, although the microhardness of the Ufi Gel Hard was affected by 2.5% CHX loading, no degradation of this property was observed. In fact, loading Ufi Gel Hard with 2.5% CHX increased KNH that may be due to the chemical conformation of poly(ethyl methacrylate) whose chemical union may be promoted by some concentration of CHX. Similar to this result was obtained in a previous study, 45 with 5% CHX group yielding higher microhardness value than the control group (0% CHX).
Concerning to flexural strength, no differences were found between the different concentration of CHX loading of the specimens made with Ufi Gel Hard or Kooliner. The fact that both direct reline resins, Ufi Gel Hard and Kooliner, were not affected by the CHX loading might be explained by their similar chemical composition, being both based on pre‑polymerized poly(ethyl methacrylate) particles.50 In a previous study45 were found similar results, since specimens of Kooliner and Ufi Gel Hard incorporated with CHX in the concentration of 1%, 2.5%, 5% and 7.5% did not evidence a negative impact on flexural strength. Nevertheless, Ufi Gel Hard with 10% CHX load should not be used for reline procedures intended to last for more than 1 month.
Probase Cold was the only resin that revealed a decrease of the flexural strength of some CHX loaded specimens since 5% CHX group had lower flexural strength than the control.
This indirect acrylic resin has a different chemical composition than the other resins studied since it is composed by pre‑polymerized poly(methyl methacrylate) particles with a distinctive structural arrangement. Moreover, unlike Kooliner and Ufi Gel Hard, Probase Cold curing cycle is accomplished under high temperature and pressure. These reasons might explain the negative influence of CHX on Probase Cold specimens. The result obtained in the Probase Cold 5% CHX group is in accordance with many other studies, 15, 44, 45, 48, 49, 56, 62 that revealed a reduction on resin flexural strength after being loaded with antimicrobial agents, although only one44 had implemented a thermal aging procedure. An inverse proportional ratio between the concentration of the antimicrobial introduced and the flexural strength values, meaning that higher amounts of drug loaded in the acrylic resins are translated in lower flexural strength values of the materials, has also been described. 48, 49, 56, 62 The physical presence of the CHX particles into the resin matrix may disturb the physical form of the polymer. 62 The decrease of flexural strength of the denture base acrylic resin may be explained by the possible increase of the intermolecular distance between the polymer chains after the incorporation of some antimicrobial monomer.49 Also, the reduced flexural strength of the denture base acrylic resin loaded with 2‑tert‑butylaminoethyl methacrylate antimicrobial monomer have been associated to the presence of a higher amount of residual monomer and a lower conversion degree of the acrylic resin. Therefore, the reduction of the flexural strength of Probase Cold 5% CHX group could be substantiated by the increase of intermolecular distance of polymer chains and the increase of residual monomer. Diminished flexural strength can result in a greater incidence of fracture when the acrylic is submitted to occlusal stress.48 Even so, in this particular situation, the 5% CHX Probase Cold group still reached a flexural strength value that is clinically accepted by the ISO 1567 standard (65 MPa).51, 56
Recent preliminary results from a microbiological study(34) established that the most effective concentration of CHX (w/w) against Candida albicans would be 2.5% for Kooliner and 5% for both Ufi Gel Hard and Probase Cold. So, it can be concluded that the proportion of 2.5% CHX for Kooliner and 5% CHX for Ufi Gel Hard may be valid because, besides being effective against Candida albicans, it did not negatively affect the mechanical properties of these direct reline acrylic resins. On the other hand, loading Probase Cold with a concentration of 5% CHX may not be advisable.
However, it is important to investigate other mechanical and physical properties of reline acrylic resins loaded with CHX, after not only thermal but also chemical aging. Although having conducted thermal aging of the specimens, more experimental studies are needed to conclude about biodegradation of acrylic reline resins exposed to the oral cavity. The biodegradation of a biomaterial can produce leachable products, which in turn may induce a series of biological responses on cells and tissues. This process may occur not only due to thermal changes but also due to exposure to saliva, chewing, breathing, chemical and dietary changes. 10 A major clinically significant consequence of acrylic based resins biodegradation is the release of potentially toxic unbound/uncured monomers or/and additives from the polymer network. The released compounds may have a toxic effect on the oral cavity. Concerning materials stability, biodegradation may induce significant changes in materials physical and mechanical properties that may ultimately lead to the failure of the material.
Conclusions
The concentrations of 1%, 2.5%, 5% and 7.5% CHX for Kooliner, 1%, 2.5%, 5%, 7.5% and 10% CHX for Ufi Gel Hard and 1% and 2.5% CHX for Probase Cold do not negatively affect the mechanical properties of the acrylic resins after a thermal aging equivalent to one month of oral environment.
References
1. Budtz‑Jorgensen E. Prosthodontics for the elderly: diagnosis and treatment. 1st edition. United States: Quintessence Publishing Co, Inc.; 1999.
2. Lyon JP, da Costa SC, Totti VMG, Munhoz MFV, de Resende MA. Predisposing conditions for Candida spp. carriage in the oral cavity of denture wearers and individuals with natural teeth. Can J Microbiol. 2006;52:462-7.
3. Figueiral MH, Azul A, Pinto E, Fonseca PA, Branco FM, Scully C. Denture‑related stomatitis: identification of aetiological and predisposing factors – a large cohort. J Oral Rehabil. 2007;34:448‑55.
4. Urban VM, De Souza RF, Galvao Arrais CA, Borsato KT, Vaz LG. Effect of the association of nystatin with a tissue conditioner on its ultimate tensile strength. J Prosthodont. 2006;15:295-9.
5. Vanden Abbeele A, De Meel H, Ahariz M, Perraudin JP, Beyer I, Courtois P. Denture contamination by yeasts in the elderly. Gerodontology. 2008;25:222-8.
6. Koray M, Ak G, Lurklu E, Issever H, Tanyeri G, Guc U. Fluconazole and/or hexetidine for management of oral candidiasis associated with denture‑induced stomatitis. Oral Dis. 2005;11:309-13.
7. Gendreau L, Loewy ZG. Epidemiology and etiology of denture stomatitis. J Prosthodont. 2011;20:251-60.
8. Harwood CL. The evidence base for current practices in prosthodontics. Eur J Prosthodont Restor Dent. 2008;16:24‑34.
9. Anusavice KJ, Chen S, Rawls HR. Phillips’ Science of Dental Materials. 12 ed. Missouri: Elsevier. 2014.
10. Bettencourt AF, Neves CB, Almeida MS, Pinheiro LM, Oliveira SA, Lopes LP, Castro MF. Biodegradation of acrylic based resins: a review. Dent Mater. 2010; 26:e171‑80.
11. Brozek R, Koczorowski R, Rogalewicz R, Voelkel A, Czarnecka B, Nicholson JW. Effect of denture cleansers on chemical and mechanical behavior of selected soft lining materials. Dent Mater. 2011;27:281-90.
12. Bettencourt AF, Feliz M, Sousa C, Gonçalves L, Neves CB. An acrylic reline resin loaded with chlorhexidine: Insights on drug release. Rev Port Estomatol Med Dent Cir Maxilofac. 2016;57:125‑31.
13. Gujan D, Berzins DW, Dhuru VB, Periathamby AR, Dentino A. Physical properties of denture base resins potentially resistant to Candida adhesion. J Prosthodont. 2007;16:465‑72.
14. Fermandes RA, Lovato‑Silva CH, Paranhos Hde F, Ito IY. Efficacy of three denture brushes on biofilm removal from complete dentures. J Appl Oral Sci. 2007;15:39‑43.
15. Casemiro LA, Gomes Martins CH, Pires‑de‑Souza Fde C, Panzeri H. Antimicrobial and mechanical properties of acrylic resins with incorporated silver‑ zinc zeolite – part I. Gerodontology. 2008;25:187‑94.
16. Salerno C, Pascale M, Contaldo M, Esposito V, Busciolano M, Milillo L, Guida A, Petruzzi M, Serpico R. Candida‑associated denture stomatitis. Med Oral Patol Oral Cir Bucal. 2011;16:e139‑43.
17. Figueiral MH, Fonseca P, Lopes MM, Pinto E, Pereira‑Leite T, Sampaio‑Maia B. Effect of denture‑related stomatitis fluconazole treatment on oral candida albicans susceptibility profile and genotypic variability. Open Dent J 2015;9:46‑51.
. Scully C, El‑Kabir M, Samaranayake LP. Candida and oral candidosis: a review. Crit Rev Oral Biol Med. 1994; 5:125-57.
19. Wilson J. The aetiology, diagnosis and management of denture stomatitis. Br Dent J. 1998;185:380-4.
20. Cross LJ, Williams DW, Sweeney CP, Jackson MS, Lewis MA, Bagg J. Evaluation of the recurrence of denture stomatitis and Candida colonization in a small group of patients who received itraconazole. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:351-8.
21. Neppelenbroek KH, Pavarina AC, Palomari Spolidorio DM, Sgavioli Massucato EM, Spolidorio LC, Vergani CE. Effectiveness of microwave disinfection of complete dentures on the treatment of Candida‑related denture stomatitis. J Oral Rehabil. 2008;35:836-46.
22. Amin WM, Al‑Ali MH, Salim N, Al‑Tarawneh SK. A new form of intraoral delivery of antifungal drugs for the treatment of denture‑induced oral candidosis. Eur J Dent. 2009;3:257-66.
23. Salim N, Moore C, Silikas N, Satterthwaite JD, Rautemaa R. Fungicidal amounts of antifungals are
24. Salim N, Moore C, Silikas N, Satterthwaite J, Rautemaa R. Candidacidal effect of fluconazole and chlorhexidine released from acrylic polymer. J Antimicrob Chemother. 2013;68:587-92.
25. Nikawa H, Jin C, Makihira S, Egusa H, Hamada T, Kumagai H. Biofilm formation of Candida albicans on the surfaces of deteriorated soft denture lining materials caused by denture cleansers in vitro. J Oral Rehabil. 2003;30:243-50.
26. Boscato N, Radavelli A, Faccio D, Loguercio AD. Biofilm formation of candida albicans on the surface of a soft denture‑lining material. Gerodontology. 2009;26:210-3.
27. Salim N, Silikas N, Satterthwaite JD, Moore C, Ramage G, Rautemaa R. Chlorhexidine‑impregnated PEM/THFM polymer exhibits superior activity to fluconazole‑impregnated polymer against Candida albicans biofilm formation. Int J Antimicrob Agents. 2013;41:193-6.
28. Bueno MG, Urban VM, Barbério GS, da Silva WJ, Porto VC, Pinto L, Neppelenbroek KH. Effect of antimicrobial agents incorporated into resilient denture relines on the Candida albicans biofilm. Oral Dis. 2015;21:57-65.
29. Bertolini MM, Portela MB, Curvelo JAR, Soares RMA, Lourenço EJV, Telles DM. Resins‑based denture soft lining materials modified by chlorhexidine salt incorporation: An in vitro analysis of antifungal activity, drug release and hardness. Dent Mater. 2014;30:793-8.
30. Garner SJ, Nobbs AH, McNally LM, Barbour ME. An antifungal coating for dental silicones composed of chlorhexidine nanoparticles. J Dent. 2015;43:362-72.
31. Malakhov A, Wen J, Zhang B, Wang H, Geng H, Chen XD, Sun Y, Yeh CK. Rechargeable anticandidal denture material with sustained release in saliva. Oral Dis. 2016;22:391-8.
32. Neppelenbroek KH, Lima JFM, Hotta J, Galitesi LL, Almeida ALPF, Urban VM. Effect of incorporation of antifungal agents on the ultimate tensile strength of temporary soft denture liners. J Prosthodont. 2018;27:177-81.
33. Salim N, Satterthwaite J, Rautemaa R, Silikas N. Impregnation with antimicrobials has an impact on degree of conversion and colour stability of acrylic liner. Dent Mater J. 2012;31:1008-13.
34. Costa J, Alexandre F, Marcelino N, Ribeiro I, Bettencourt A, Portugal J, Neves CB. Drug Incorporation of acrylic resins – Microbiological and release studies”. J Dent Res. 2017;96(special issue B):#0048.
35. MacNeill S, Rindler E, Walker A, Brown AR, Cobb CM. Effects of tetracycline hydrochloride and chlorhexidine gluconate on Candida albicans. An in vitro study. J Clin Periodontol. 1997;24:753-60.
36. Suci PA, Tyler BJ. Action of chlorhexidine digluconate against yeast and filamentous forms in an early‑ stage Candida albicans biofilm. Antimicrob Agents Chemother. 2002;46:3522-31.
37. Pusateri CR, Monaco EA, Edgerton M. Sensitivity of Candida albicans biofilm cells grown on denture acrylic to antifungal proteins and chlorhexidine. Arch Oral Biol. 2009;54:588-94.
38. da Silva PM, Acosta EJ, Pinto LR, Graeff M, Spolidorio DM, Almeida RS, Porto VC. Microscopical analysis of Candida albicans biofilms on heat‑ polymerised acrylic resin after chlorhexidine gluconate and sodium hypochlorite treatments. Mycoses. 2011;54:712-7.
39. Iqbal Z, Zafar MS. Role of antifungal medicaments added to tissue conditioners: A systematic review. J Prosthodont Res. 2016;60:231-9.
40. Lamfon H, Porter SR, McCullough M, Pratten J. Susceptibility of Candida albicans biofilms grown in a constant depth film fermentor to chlorhexidine, fluconazole and miconazole: a longitudinal study. J Antimicrob Chemother. 2004;53:383-5.
41. Redding S, Bhatt B, Rawls HR, Siegel G, Scott K, Lopez‑Ribot J. Inhibition of Candida albicans biofilm formation on denture material. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:669-72.
42. Ryalat S, Darwish R, Amin W. New form of administering chlorhexidine for treatment of denture‑induced stomatitis. Ther Clin Risk Manag. 2011;7:219-25.
43. Marcelino NIF, Barreiros MCS, Bettencourt AF, Neves CB. Efeito da incorporação de clorexidina em resinas de rebasamento – Estudos de libertação. Rev Port Estomatol Med Dent Cir Maxilofac. 2015;56(S1):14.
44. Sousa C, Costa J, Matos A, Bettencourt, Portugal J, Neves CB. Efeito da incorporação de clorexidina nas propriedades de resinas acrílicas de rebasamento. Rev Port Estomatol Med Dent Cir Maxilofac. 2014;55(S1):e23‑4.
45. Lacerda S, Portugal J, Neves CB. Efeito da incorporação de clorexidina nas propriedades mecânicas de resinas de rebasamento. Rev Port Estomatol Med Dent Cir Maxilofac. 2015;56(S1):e21.
46. Urban VM, Machado AL, Oliveira R V, Vergani CE, Pavarina AC, Cass QB. Residual monomer of reline acrylic resins. Effect of water‑bath and microwave post‑polymerization treatments. Dent Mater. 2007;23:363-8.
47. Urban VM, Seó RS, Giannini M, Arrais CA. Superficial distribution and identification of antifungal/antimicrobial agents on a modified tissue conditioner by SEM‑EDS microanalysis: A preliminary study. J Prosthodont. 2009;18:603-10.
48. Cunha TR, Regis RR, Bonatti MR, de Souza RF. Influence of incorporation of fluoroalkyl methacrylates on roughness and flexural strength of a denture base acrylic resin. J Appl Oral Sci. 2009;17:103-7.
49. Rodriguez LS, Paleari AG, Giro G, de Oliveira Junior NM, Pero AC, Compagnoni MA. Chemical characterization and flexural strength of a denture base acrylic resin with monomer 2‑tert‑butylaminoethyl methacrylate. J Prosthodont. 2013;22:292‑7.
50. Arima T, Murata H, Hamada T. Properties of highly cross‑linked autopolymerizing reline acrylic resins. J Prosthet Dent. 1995;73:55-9.
51. International Standard ISO Specification 20795‑1: Dentistry‑ Base polymers‑Part 1: Denture base polymers. International Organization for Standardization 2013. 1st edition, Geneva, Switzerland.
52. Neves CB, Lopes LP, Ferrao HF, Miranda JP, Castro MF, Bettencourt AF. Ethanol postpolymerization treatment for improving the biocompatibility of acrylic reline resins. Biomed Res Int. 2013;2013:485246.
53. Sodagar A, Bahador A, Khalil S, Shahroudi AS, Kassaee MZ. The effect of TiO2 and SiO2 nanoparticles on flexural strength of poly (methyl methacrylate) acrylic resins. J Prosthodont Res. 2013;57:15‑9.
54. Arima T, Murata H, Hamada T. Analysis of composition and structure of hard autopolymerizing reline resins. J Oral Rehabil. 1996;23:346‑52.
55. Diaz‑Arnold AM, Dunne J T, Jones AH. Microhardness of provisional fixed prosthodontic materials. J Prosthet Dent. 1999;82:525-8.
56. Paleari AG, Marra J, Pero AC, Rodriguez LS, Ruvolo‑Filho A, Compagnoni MA. Effect of incorporation of 2‑tert‑butylaminoethyl methacrylate on flexural strength of a denture base acrylic resin. J Appl Oral Sci. 2011;19:195-9.
57. Al‑Haddad A, Vahid Roudsari R, Satterthwaite JD. Fracture toughness of heat cured denture base acrylic resin modified with Chlorhexidine and Fluconazole as bioactive compounds. J Dent. 2014;42:180-4.
58. Barreiros MCS, Marcelino NIF, Bettencourt AF, Neves CB. Incorporação de clorexidina em resinas de rebasamento – Propriedades de superfície. Rev Port Estomatol Med Dent Cir Maxilofac. 2015;56(S1):14.
59. Palmer DS, Barco MT, Billy EJ. Temperature extremes produced orally by hot and cold liquids. J Prosthet Dent. 1992;67:325‑7.
60. Gale MS, Darvell BW. Thermal cycling procedures for laboratory testing of dental restorations. J Dent 1999;27:89‑99.
. Cao Z, Sun X, Yeh CK, Sun Y. Rechargeable infection‑responsive antifungal denture materials. J Dent Res. 2010;89:1517-21.
62. Addy M, Handley R. The effects of the incorporation of chlorhexidine acetate on some physical properties of polymerized and plasticized acrylics. J Oral Rehabil. 1981;8:155-63.
Cristina Bettencourt Neves
Correio eletrónico: cristina.neves@fmd.ulisboa.pt
Ethical disclosures
Protection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of data. Theauthors declare that no patient data appear in this article.
Right to privacy and informed consent. The authors declare that no patient data appear in this article.
Conflict of interest
The authors have no conflicts of interest to declare.
Article history:
Received 8 August 2018
Accepted 18 November 2018
Available online 30 November 2018
